* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 10
Copper in heat exchangers wikipedia , lookup
Intercooler wikipedia , lookup
Cogeneration wikipedia , lookup
Solar air conditioning wikipedia , lookup
Building insulation materials wikipedia , lookup
Hyperthermia wikipedia , lookup
R-value (insulation) wikipedia , lookup
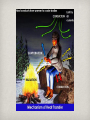
Chapter 10 Heat transfer & Change of Phase 3 methods of heat transfer 1. Conduction 2. Convection 3. Radiation 10.1- Conduction Conduction is the transfer of heat energy in a substance caused by the collision of the particles in the substance. Good and bad conductors Solids whose atoms or molecules have loosely held electrons are GOOD conductors of heat. Silver is the BEST conductor of heat. Wood, wool, paper, cork, and plastic foam are poor conductors of heat, since their electrons are firmly attached to their molecules. Materials like these are called insulators. fun fact A “firewalker” can walk across hot wooden coals, because wood is such a poor conductor of heat. air keeps us warm! Air is a very poor conductor. Many items that are good insulators (and poor conductors) are this way because they have many air spaces. snow and heat? Snow is a poor conductor of heat. Many animals use this to their advantage when burrowing in the snow to help reduce the loss of their own body heat. insulation in homes Insulation in homes slows the rate at which heat flows. It keeps warm air in the house longer in the winter, and keeps heat from the outside out of the inside during the summer. 10.2- convection Liquids and gases transfer heat mainly by convection, which is the transfer by motion of a fluid in currents. convection in fluids Convection occurs in all fluids. As the fluid is heated, the molecules close to the heat source begin moving faster and spread out, becoming less dense. Due to the decreased density, the warmer fluid rises up, while cooler, denser fluid sinks to the bottom. • Convection currents in the air produce winds. 10.3- Radiation Radiation is the transfer of thermal energy by mean of electromagnetic waves. An example of radiation is the sun warming the Earth. Energy transferred by radiation is called radiant energy. the electromagnetic spectrum EM waves include radio waves, infrared waves, and visible light waves. The differences between them are the wavelengths. The wavelength of radiation is related to the frequency of radiation. Frequency is the rate of vibration of a wave source. The lower the frequency, the longer the wave. All substances above absolute zero emit radiant energy. The average frequency of the radiant energy is directly proportional to the temperature of the emitter. F ~ T The sun is hot, therefore, it emits a higher frequency energy. The Earth is cool, therefore, it emits a lower frequency (infrared) energy, called terrestrial radiation. All objects continually emit radiant energy in a mixture of frequencies. In addition to emitting energy, all objects absorb energy. Good emitters are also good absorbers. An object that absorbs radiant energy looks dark. If it absorbs all the radiant energy on it, it looks black. A black container full of hot water will cool faster than a white or shiny one. painting your house a light color is good because: Lightly colored buildings reflect much of the incoming radiant energy. This helps them stay cooler in the summer. Lightly colored objects are also poor emitters, so they retain much of their internal energy during the winter, and stay warmer. Phase Changes phases of matter 1. Solid 2. Liquid 3. Gas 4. Plasma: occurs when molecules from a gas break into ions and electrons. (Illuminating gas found in fluorescent and other vapor lamps) Phases of Matter Phase Definite Volume? Definite Shape? Solid Yes Yes Liquid Yes No (takes the shape of its container) Gas No No (takes the shape of its container) 10.5- Evaporation and Sublimation Evaporation: Process by which a liquid changes into a gas. Sublimation: A solid evaporates directly to a gas. (Reason why ice cubes left in a freezer for a long time get smaller). Melting and Freezing • Melting: When an object goes from a solid to a liquid • Freezing: When an object goes from a liquid to a solid 10.6- Condensation Opposite of evaporation. Condensation is the changing of a gas to a liquid. Deposition • Deposition: Changing from a gas directly to a solid • Ex: Frost forming on a leaf 10.7 & 10.8 Boiling, Freezing, and Melting Points Boiling Point: The temperature at which a substance boils Melting Point: The temperature at which a substance melts. Freezing Point: The temperature at which a substance freezes. 10.9 Heat of Vaporization & Fusion Heat of fusion: the amount of energy needed to change any substance from solid to liquid (and vice versa). For water: 335 joules per gram Heat of vaporization: the amount of energy needed to change any substance from liquid to gas (and vice versa). For water: 2255 joules per gram Phase Change Triangle Gains Heat Energy Loses Heat Energy Solid Gas Liquid