* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atomic Models - South Houston High School
Survey
Document related concepts
Transcript
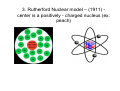
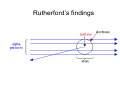
Atomic Models Chapter 5 1. John Dalton – Billiard Ball Model John Dalton – English Chemist – 1803 Dalton’s postulates 1. All elements are composed of tiny indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. 2. Understand Conclusions Show that you understand the conclusions used in the development of modern atomic theory by describing how Democritus’s idea of the atom was different from Dalton’s. Dalton’s postulates • Dalton’s postulates are illustrated below. 3. Interpret Diagrams How does a mixture of atoms of different elements differ from a compound? Self-check: • To demonstrate that you understand the conclusions used in the development of modern atomic theory, identify the statement that is NOT part of Dalton’s postulates. • A All elements are composed of atoms. • B Atoms of the same element are alike. • C Atoms are always in motion. • D Atoms that combine do so in simple wholenumber ratios. More Atomic Models 2. Thomson model – (1897) - Plum-pudding model -a ball of positive charge containing a number of electrons (ex: chocolate chip cookie dough) Self - check To show that you understand the experimental design used in the development of atomic theory, summarize the evidence used by Thomson to argue that cathode rays consist of negatively charged particles. 3. Rutherford Nuclear model – (1911) center is a positively - charged nucleus (ex: peach) Rutherford’s findings Self-check Compare Rutherford’s expected outcome of the gold-foil experiment with the actual outcome to show that you understand experimental design used in the development of modern atomic theory. Self-check Demonstrate that you understand the conclusions used in the development of modern atomic theory by describing the evidence that led Rutherford to conclude that an atom is mostly empty space. More Atomic Models 4. Bohr planetary model – (1913) electrons travel in definite orbits around the nucleus (ex: planets around the sun) Self-check • Fill in the blanks to show that you understand the experimental design used in the development of modern atomic theory: “According to Bohr’s atomic theory, the atomic emission spectrum of hydrogen results when atoms of hydrogen emit ____________ as ____________ move from a ____________ energy level to a ____________ energy level.” Self-check • To demonstrate that you understand the conclusions used in the development of modern atomic theory, identify how Bohr’s nuclear atom differs from Rutherford’s nuclear atom. • A Bohr’s model proposed that electrons occupy orbits of different shapes. • B Rutherford’s model explained the physical and chemical properties of elements, whereas Bohr’s model only explained the physical properties. • C Bohr’s model states that electrons occupy orbits with fixed energy levels. • D In Bohr’s model, electrons occupy space within a “cloud” surrounding the nucleus. More atomic models 5. Quantum model – electrons can be found in certain regions of space around the nucleus called orbitals Even though the quantum model is the most recent, we still use the Bohr model to illustrate the atom in high school chemistry! Some specifics about the Bohr model: The Octet Rule Atoms are most stable when they have 8 electrons in their outer shell 1st energy level holds 2 electrons 2nd energy level holds 8 3rd usually holds 8 (to a max of 18) And so on…