* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download bacteriophage and viruses-part-ii-study material
Elsayed Elsayed Wagih wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
Canine distemper wikipedia , lookup
Canine parvovirus wikipedia , lookup
Orthohantavirus wikipedia , lookup
Hepatitis B wikipedia , lookup
Influenza A virus wikipedia , lookup
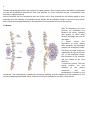
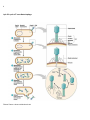
1 VIRAL MULTIPLICATION Virus particle outside host cell have no independent metabolic activity and are incapable of reproduction by the process characteristic of other microorganisms. Multiplication takes place by replication in which the viral protein and nucleic acid components are reproduced within susceptible host cell. The essential features common to the replication cycle of all viruses include entry into host cell, intracellular reproduction to produce progeny virion, escape of these into the environment and survival there. All viruses that are obligate intracellular parasitic pass through a superficially similar cycle, but there are fundamental differences. Viruses do not reproduce by division rather they are replicated by a process in which all molecular components are synthesized separately. Then, these are assembled into intact virion. For a specific virus to replicate within host cell: 1. The host cell must be permissive and the virus must be compatible with the host cell, 2. The host cell must not degrade the virus, 3. The viral genome must possess the information for modifying the normal metabolism of the host cell, 4. The virus must be able to use the metabolic capabilities of the host cell to produce new virus particles containing replicated copies of viral genome. The steps of viral infection and replication are: a) Adsorption, b) Penetration and uncoating, c) Component replication and biosynthesis, d) Assembly and e) Release. Multiplication of T-Even Phages, Animal viruses and Plant viruses: The infection cycle of Teven bacteriophage lasts about 20 minutes, culminating in lysis (bursting-open) of the host cell, E. coli. The whole process can be classified into, (i) adsorption or infection. (ii) Penetration or injection, (iii) the eclipse or the latent period (iv) maturation and (v) lysis or release. (i) Adsorption or infection: Attachment of the virus particle onto the surface of the host cell is adsorption or infection. The virus particles possess one or more proteins on the outside that interact with cell surface components called receptors; the receptors are normal surface components of the host (e.g., proteins, carbohydrates, glycoproteins, lipids, lipoproteins, etc.). In fact, these are the receptors that determine 2 which cells will be susceptible to infection. In the absence of the receptor site, the virus cannot adsorb and hence cannot infect. If the receptor site is altered, the host may become resistant to virus infection. The animal virus particles get absorbed to the plasma membrane of the host cells by binding to specific sites where the receptor proteins (usually glycoproteinsl are situated. The presence of these receptor proteins is crucial in the viral infection and it may determine host resistance or susceptibility. The receptor proteins are usually surface proteins necessary for the host cell, as these proteins are also receptors for hormones and other important molecules which get into the cell and are essential to the cell's function. The virus mimics these essential molecules and manages to get into the cell by endocytosis. Many host receptor proteins are related to immunolgobulins. For example HIV CD4 receptor, and the polio ICAM (intercellular adhesion molecule) receptor. Envelope glycoproteins may also be involved in adsorption in enveloped viruses. The herpes simplex virus has two glycoproteins that are involved in adsorption. Spikes of some enveloped viruses also play similar roles (myxovirus). For example, the influenza virus has two kinds of spikes, haemagglutinin, and neutraminidase. The haemogglutinin (H spike) attach to the host cell receptor site and recognize siatic acid (N-acetyl neuraminic acid). The N spike (Neuraminidase) helps the virus in penetrating the nasal and respiratory tract secretions by degrading mucosal polysaccharides. However the receptor sites vary from person to person. Plant viruses enter their host through the cell wall. (ii) Penetration or Injection: After the tail fibres get adsorbed, an enzyme-system is supposed to make a pore or hole in the cell wall of the host. It is believed that the enzyme-system consists of a phage-lysozyme, which is synthesized during the multiplication of the parent phage inside the host cell and its molecules remain attached to the extreme tip of the tail-fibres of the new progeny phages. In the T-phage penetration is activated when: i) the tail fibers of the virus attach to the cell and hold the tail firmely against cll wall. ii) The sheath contracts driving the core into cell through the cell wall and cell membrane , iii) The virus injects its DNA the way a syringe injects. This enzymes system becomes active when the released phage particles infect the new host cell. However, the tail-fibres attached on the surface of the host cell bend to bring the end-plate in contact with the cell wall surface. Now, the protein sheath of the tail longitudinally contracts pushing the central tubular core through the pore inside the wall of the host cell and the phage DNA molecule is released or injected into the cytoplasm. After the DNA is released, the empty protein coat becomes of no use. In animal viruses direct penetration or fusion with plasma membrane occurs during penetration.Some nonenveloped viruses such as the polio virus, undergo changes in capsid structure on adsorption to the plasma membrane, and release only their nucleic acids intothe host cell. In the paramyxoviruses, and some other enveloped viruses, the capsids fuse with host cell plasma membrane. Fusion occurs between the envelope glycoprotins and the host plasma membrane proteins. Then the membrane lipids rearrange forming a proteinaceous fusion pore. The nucelocapsid enters the host cell where uncoating take place. Endocytosis: Enveloped viruses may enter the host cell in another way. The virions attach to specialized regions on the membrane coated on the cytoplasmic side with protein clathrin. The coated regions pinch off to form coated vesicles filled with virus particles. The vesicles fuse with lysosomes after the coating is removed. The lysosomal enzymes help in theuncoating process. 3 Uncoating: This is a process of separation of viral nucleic acid from the protein coat. This process is not fully understood. In some viruses the coating is done by lysosomal enzymes of the host cell which degrade protein coat and make the nucleic acid free in cytoplasm. In Pox virus the viral DNA synthesizes a specific protein after infection. Thus it varies with virus groups. Plant viruses penetrate host cells through transient pores (ectodesmata) which protrude through the cell wall at intervals and communicate to the exterior to the cells. Uncoating occurs inside the plant cells. (iii) The Eclipse or the Latent Period/ Component replication and biosynthesis: When the DNA molecule is released in the host cytoplasm, it is not degraded by the nuclease enzymes of the host cell. It has been studied, particularly in T4, phage, that the phage DNA contains glucosylated hydroxymethyl cytosine instead of cytosine, which prevents the nucleases of the bacterium from degrading the phage DNA. The phage DNA, first makes the host cell immune against infection by genetically similar phage particles. Secondly, it immediately takes over the charge of the cell machinery and suppresses all cellular activities such as synthesis of cellular DNA, RNA, proteins, etc. This is the parasitism of a virus at the genetic level. Transcription occurs in several stages leading to the formation of immediate early, delayed early and late gene products. i) The bacterial m-RNA and protein stop being synthesized within a few minutes after the entry of phage DNA. ii) Bacterial DNA is quickly degrade to small fragments and the nucleoids fragments of bacterium become dispersed. iii) Some phage m-RNA is made immediately after infection and the amount of phage DNA increases after the brief delay. iv) Virus specific proteins appear somewhat late followed by appearance of organized capsid precursors and resulting in the formation of mature infectious capsid. 4 Immediate early phage genes are translated using the existing bacterial RNA polymerase and these genes code for nucleases that break down host DNA and for the enzymes that alter bacterial RNA polymerases . Delayed early genes code for phage enzymes which produce unique phage DNA constituent such as 5-hydroxy methyl cytosine, which glycosates these nucleotides and which destroy cytosine deoxynucleotides so that no bacterial cytosine will be incorporated into phage DNA. These alterations enable the phage to survive because bacterial restriction enzymes are unable to degrade phage DNA modified by the substitution of 5-hydroxymethyl cytosine. Delayed early genes also code for a altered DNA polymerase that will transcribe the late genes. Late genes products include the structural components of new phage particles such as heads, tail fibers and also include lysozyme which will lyse the bacterial cell releasing the mature virion. The virulent animal viruses arrest all the functions of the host cell such as DNA,RNA and protein synthesis. The virus DNA replication usually takes place in the host nucleus using host DNA polymerase-II, except in the poxviruses (such as vaccinia) whose genomes replicate in the cytoplasm. In most viruses, early transcription occurs using host enzymes (polymerases) except in poxvirus where early mRNA is transcribed by a viral polymerase. The exact mechanisms for the replication of new copies of the viral genome vary with different types of viruses i.e; whether their nucleic acid is DNA or RNA and their type of strands. Generally the double stranded DNA viruses include those capable of using host cell polymerases and RNA viruses require viral coded polymerases. (iv) Maturation/ Assembly: Assembly of the various components to constitute a new phage particle within the host cell is called maturation. Head the tail formation start separately, the protein components aggregate around the DNA and form the head of the phage. End-plate is formed first followed by the formation of tubular core. Tail fibres are formed later. Hundreds (about 200) of new phage particles are produced from each bacterium by the time of lysis. 5 The late expressing genes direct the synthesis of capsid proteins. Once enough protein and DNA are synthesized the two will spontaneous assemble to form virus particles, as in the case plant viruses. In icosahedral virus assembly, it appears that the empty procapsids are first formed and then the nucleic acid is then inserted into the empty capsid in some unknown way. The assembly of enveloped viruses follows the same pattern except in the case of pox viruses which follow more complicated pattern and assemble in the cytoplasm rather than the nucleus. v) Release. After all components of virion particles are assembled, the bacterial cell bursts, releasing new phages to infect other bacteria and begin the cycle all over again. In animal viruses ,the mechanism of virion release differ between non-enveloped (naked) and enveloped viruses. The virions of naked viruses are released by the lyse of the host cell. In the enveloped viruses, the formation of the envelope and the release of the virus particle is a concurrent process. The viral capsid proteins are first attached to the plasma membrane, and the nucleocapsid is formed on the membrane. The nucelocapsid is released by membrane budding, and the capsids carry the membrane in the process of budding andreleased. Active filaments of the host cytoskeleton can aid in virion release. 6 Lytic life cycle of T-even Bacteriophage Picture Source: classes.midlandstech.edu 7 The One-Step Growth Experiment The development of the one-step growth experiment in 1939 by Max Delbrück and Emory Ellis marks the beginning of modern bacteriophage research. In a one-step growth experiment, the reproduction of a large phage population is synchronized so that the molecular events occurring during reproduction can be followed. A culture of susceptible bacteria such as E. coli is mixed with bacteriophage particles, and the phages are allowed a short interval to attach to their host cells. The culture is then greatly diluted so that any virus particles released upon host cell lysis will not immediately infect new cells. This strategy works because phages lack a means of seeking out host cells and must contact them during random movement through the solution. Thus phages are less likely to contact host cells in a dilute mixture. The number of infective phage particles released from bacteria is subsequently determined at various intervals by a plaque count. A plot of the bacteriophages released from host cells versus time shows several distinct phases. During the latent period, which immediately follows phage addition, there is no release of virions. This is followed by the rise period or burst, when the host cells rapidly lyse and release infective phages. Finally, a plateau is reached and no more viruses are liberated. The total number of phages released can be used to calculate the burst size, the number of viruses produced per infected cell. The latent period is the shortest time required for virus reproduction and release. During the first part of this phase, host bacteria do not contain any complete, infective virions. This can be shown by lysing them with chloroform. This initial segment of the latent period is called the eclipse period because the virions were detectable before infection but are now concealed or eclipsed. The number of completed, infective phages within the host increases after the end of the eclipse period, and the host cell is prepared for lysis. The one-step growth experiment with E. coli and phage T2 provides a well-studied example of this process. When the experiment is carried out with actively growing cells in rich medium at 37°C, the growth curve plateau is reached in approximately 30 minutes. Bacteriophage reproduction is an exceptionally rapid process, much faster than animal virus reproduction, which may take hours. This sequence of events initiated by the infection of the phage nucleic acid and culminating in the release of newly synthesized virion is termed viral multiplication cycle and can be used to follow the kinetics of synthesis of viral components as well as intact virions LYSOGENY Lysogeny, or the lysogenic cycle, is one of two methods of viral reproduction (the lytic cycle is the other). Lysogeny is characterized by integration of the bacteriophage nucleic acid into the host bacterium's genome. The newly integrated genetic material, called a prophage can be transmitted to daughter cells at each subsequent cell division, and a later event (such as UV radiation) can release it, causing proliferation of new phages via the lytic cycle. The distinction between lysogenic and lytic cycles is that the spread of the viral DNA occurs through the 8 usual prokaryotic reproduction, while the lytic phage is spread through the production of thousands of individual phages capable of surviving and infecting other bacterium. The process of lysogeny: i) After penetration the viral chromosome directs the production of repressor protein that specifically binds to the viral chromosome and turn off replication of viral DNA. ii) The repressed viral DNA then integrates into and becomes the physical part of the host chromosome. The integrated virus DNA is now called a prophage. iii) The virus DNA replicates whenever the bacterial chromosomes doubles so all the progeny cells inherit one copy of the prophage in the chromosome and this carry the potential for producing the temperate phage. iv) An induction occurs occasionally when prophage detaches from bacterial DNA, produces progeny viruses eventually lysing the host cells. v) Induction occurs spontaneously but its frequency in increased by irradiation with UV light. Certain types of viruses replicate by the lysogenic cycle, but also partly by the lytic cycle (mixed cycles). Some DNA phages, called temperate phages, only lyse a small fraction of bacterial cells; in the remaining majority of the bacteria, the phage DNA becomes integrated into the bacterial chromosome and replicates along with it. In this lysogenic state, the information contained in the viral nucleic acid is not expressed. The model organism for studying lysogeny is the lambda phage. Roughly 50-60 nucleotides are taken out of the lysogenic pathway and used. Advantages of Lysogeny to the Bacterial Cells: i) The cells get protection from the lytic infection by the viruses of the same type as prophage. ii) Bacteriophages may bring in new genes either by transduction or by lysogenic conversion .These new genes may increase the bacterial ability to survive .E.g. Resistance to antibiotics. Medical Use of Virulent Phage: The primary uses of bacteriophages are: i) the identification of bacterial strains, ii) used as genetic model in molecular biology. The lytic phages have been used in the detection and identification of pathogenic bacteria. Strains of bacteria may be characterized by their resistance and susceptibility to lysis by specific virulent phage. The resulting pattern of lysis from visible plaques on a lawn of bacterial growth by different phages gives an indication to the identification of strain. Picture source: classes.midlandstech.edu VIRUS CLASSIFICATION In all viral taxonomic individual viruses are grouped by: i) The nucleic acid they contain ( DNA or RNA ) ii) Single or double strandedness of nucleic acid iii) Capsid morphology iv) Presence or absence of envelope. v)Host range. 9 In some single stranded RNA viruses, the chromosome also serve as viral m-RNA termed as plus strand and while in others transcribe RNA , complementary to virus NA is termed as minus strand RNA. Animal, plant and bacterial viruses are usually classified separately. Animal viruses are often grouped into families, genera and species which are given Latin names but English names are also used. A taxonomy employing Latin family names have also been proposed for bacterial viruses but this system is rarely use. Plant viruses are grouped on the basis of the structure of virion, whether they contain DNA or RNA and its mode of transmission. In most cases the groups of plant viruses are named after a prominent representative. E.g. A group of plant virus closely related to Tobacco mosaic is termed the Tobacco group. Viruses have been traditionally named by adding the word 'virus' after the disease caused in the major host, e.g. poliovirus, the causative agent of poliomyelitis. Classification of Viruses on the basis of differentiations in their transcription process Group Description I II III IV V VI VII Double stranded DNA genome Genome replication:dsDNA→ dsDNA mRNA synthesis: dsDNA→mRNA Single stranded DNA genome Genome replication:ssDNA→ dsDNA→ ssDNA mRNA synthesis: ssDNA→ dsDNA→ mRNA Double stranded RNA genome Replication: dsRNA→ssRNA→RNA mRNA synthesis: dsRNA→mRNA Plus stranded RNA genome Replication:+RNA→-RNA→+RNA mRNA synthesis::+RNA→=mRNA Negative stranded RNA genome Replication:-RNA→+RNA→-RNA mRNA synthesis::-RNA→=mRNA Single stranded RNA genome Replication:ssRNA→dsDNA→ssRNA mRNA synthesis::ssRNA→dsDNA→mRNA Double stranded gaped DNA genome Replication: gapped dsDNA → dsDNA→+RNA→-DNA → gapped dsDNA mRNA synthesis: gapped dsDNA →dsDNA →mRNA Bacteriophages were named after laboratory code symbols e,g. φX174, P22, T7 etc. Holmes (1948) followed the Linnaeus system of binomial nomenclature. Viruses were grouped under the order Virales, which was divided into three suborders: Phaginae - infecting bacteria. Phytophaginae - infecting plants. Zoophaginae - infecting animals. 10 Classification by the Provisional committee on nomenclature of Viruses ( Linnaeus classification scheme with nomenclature). Phylum: Vira Subphylum: Deoxy vira ( DNA virus) Class: Deoxyhelica (Helical Symmetry) Order: Chitoviraes ( In Greek: Chitn – tunica or envelope) Family: Poxviridae. Phylum:Vira Subphylum: Deoxy vira ( DNA virus) Class: Deoxcubica (Cubical Symmetry) Order: Haplovirales Family: Microviridae-12 capsomeres. Phylum: Vira Sub phyum:Ribovira Class: Ribo helica (Helical Symmetry) Order:Rhabdovirales Suborder:Rigidovirales Family:Protovidae. Suborder: Flexiviridales- Plant viruses Family: Leptoviridae 10-11 nm Phylum: Vira Sub phyum:Ribovira Class: Ribo cubica (cubical Symmetry) Order:Gymnovirales Family:Napoviridae Properties that determine genera within family include i)capsomere: structure, antigenic properties, molecular weight, ii)Capsid: number of capsomeres, antigenic properties, reaction to heat, pH, other physical and chemical agents. Additional features for generic and species differentiation include data on envelope or mantle enzymes, mode of development, sensitivity to interferon, specificity for host virulence and clinical effect. Family names end in –viridae, subfamily-virinae, genera like specis-virus. 12 Typical Classification of Animal and Plant Viruses on the basis of their inherent Properties Nucleic Capsid Acid Symmetry RNA RNA RNA RNA DNA DNA DNA Icasohedral Icasohedral Helical Helical Icasohedral Icasohedral Complex Presence of Envelope + + + Coat Virion size in nm 28 35-80 175-300 80-120 50 180-200 230-300 Family or Group Typical Member Poty virus Toga viridae Tobamo virus Orthomyxoviridae Cauliovirus Herpes viridae Poxviridae Turnip yellow mosaic virus Napha virus Tobacco mosaic virus Influenza virus Cauliflower mosaic virus Herpes virus Pox virus CULTIVATION OF VIRUSES viruses cannot be cultured in the same way as bacteria and eukaryotic microorganisms. For many years researchers have cultivated animal viruses by inoculating suitable host animals or embryonated eggs—fertilized chicken eggs incubated about 6 to 8 days after laying .To prepare the egg for virus cultivation, the shell surface is first disinfected with iodine and penetrated with a small sterile drill. After inoculation, the drill hole is sealed with gelatin and the egg incubated. Viruses may be able to reproduce only in certain parts of the embryo; consequently they must be injected into the proper region. For example, the myxoma virus grows well on the chorioallantoic membrane, whereas the mumps virus prefers the allantoic cavity. The infection may produce a local tissue lesion known as a pock, whose appearance often is characteristic of the virus. More recently animal viruses have been grown in tissue (cell) culture on monolayers of animal cells. This technique is made possible by the development of growth media for animal cells and by the advent of antibiotics that can prevent bacterial and fungal contamination. A layer of animal cells in a specially prepared petri dish is covered with a virus inoculum, and the viruses are allowed time to settle and attach to the cells. The cells are then covered with a thin layer of agar to limit virion spread so that only adjacent cells are infected by newly produced virions. As a result localized areas of cellular destruction and lysis called plaques often are formed and may be detected if stained with dyes, such as neutral red or trypan blue, that can distinguish living fromdead cells. 13 Viral growth does not always result in the lysis of cells to form a plaque. Animal viruses, in particular, can cause microscopic or macroscopic degenerative changes or abnormalities in host cells and in tissues called cytopathic effects Cytopathic effects may be lethal, but plaque formation from cell lysis does not always occur. Bacterial viruses or bacteriophages (phages for short) are cultivated in either broth or agar cultures of young, actively growing bacterial cells. So many host cells are destroyed that turbid bacterial cultures may clear rapidly because of cell lysis. Agar cultures are prepared by mixing the bacteriophage sample with cool, liquid agar and a suitable bacterial culture. The mixture is quickly poured into a petri dish containing a bottom layer of sterile agar. After hardening, bacteria in the layer of top agar grow and reproduce, forming a continuous, opaque layer or “lawn.” Wherever a virion comes to rest in the top agar, the virus infects an adjacent cell and reproduces. Eventually, bacterial lysis generates a plaque or clearing in the lawn, plaque appearance often is characteristic of the phage being cultivated. Plant viruses are cultivated in a variety of ways. Plant tissue cultures, cultures of separated cells, or cultures of protoplasts may be used. Viruses also can be grown in whole plants. Leaves are mechanically inoculated when rubbed with a mixture of viruses and an abrasive such as carborundum. When the cell walls are broken by the abrasive, the viruses directly contact the plasma membrane and infect the exposed host cells. (The role of the abrasive is frequently filled by insects that suck or crush plant leaves and thus transmit viruses.) A localized necrotic lesion often develops due to the rapid death of cells in the infected area . Even when lesions do not occur, the infected plant may show symptoms such as changes in pigmentation or leaf shape. Some plant viruses can be transmitted only if a diseased part is grafted onto a healthy plant. VIRUS (ENUMERATION OF VIRUSES) ASSAYS: The quantity of viruses in a sample can be determined either by counting particle numbers or by measurement of the infectious unit concentration. Although most normal virions are probably potentially infective, many will not infect host cells because they do not contact the proper surface site. Thus the total particle count may be from 2 to 1 million times the infectious unit number depending on the nature of the virion and the experimental conditions. Despite this, both approaches are of value. Virus particles can be counted directly with the electron microscope. In one procedure the virus sample is mixed with a known concentration of small latex beads and sprayed on a coated specimen grid. The beads and virions are counted; the virus concentration is calculated from these counts and from the bead concentration. This technique often works well with concentrated preparations of viruses of known morphology. Viruses can be concentrated by centrifugation before counting if the preparation is too dilute. However, if the beads and viruses are not evenly distributed (as sometimes happens), the final count will be inaccurate. the hemagglutination assay. Many viruses can bind to the surface of red blood cells . If the ratio of viruses to cells is large enough, virus particles will join the red blood cells together, forming a network that settles out of suspension or agglutinates. In practice, red blood cells are mixed with a series of virus preparation dilutions and each mixture is examined. The hemagglutination titer is the highest dilution of virus (or the reciprocal of the dilution) that still causes hemagglutination. This assay is an accurate, rapid method for determining the relative quantity of viruses such as the influenza virus. If the actual number of viruses needed to cause hemagglutination is determined by another technique, the assay can be used to ascertain the number of virus particles present in a sample. A variety of assays analyze virus numbers in terms of infectivity, and many of these are based on the same techniques used for virus cultivation. For example, in the plaque assay several dilutions of bacterial or animal viruses are plated out with appropriate host cells. When the number of viruses plated out are much fewer than the number of host cells available for infection and when the viruses are distributed evenly, each plaque in a layer of bacterial or animal cells is assumed to have arisen from the reproduction of a single virus particle. Therefore a count of the plaques produced at a particular dilution will give the number of infectious virions or plaque-forming units (PFU), and the concentration of infectious units in the 14 original sample can be easily calculated. Suppose that 0.10 ml of a 10–6 dilution of the virus preparation yields 75 plaques. The original concentration of plaque-forming units is PFU/ml _ (75 PFU/0.10 ml)(106) _ 7.5 _ 108. Viruses producing different plaque morphology types on the same plate may be counted separately. Although the number of PFU does not equal the number of virus particles, their ratios are proportional: a preparation with twice as many viruses will have twice the plaque-forming units. The same approach employed in the plaque assay may be used with embryos and plants. Chicken embryos can be inoculated with a diluted preparation or plant leaves rubbed with a mixture of diluted virus and abrasive. The number of pocks on embryonic membranes or necrotic lesions on leaves is multiplied by the dilution factor and divided by the inoculum volume to obtain the concentration of infectious units. When biological effects are not readily quantified in these ways, the amount of virus required to cause disease or death can be determined by the endpoint method. Organisms or cell cultures are inoculated with serial dilutions of a virus suspension. The results are used to find the endpoint dilution at which 50% of the host cells or organisms are destroyed . The lethal dose (LD50) is the dilution that contains a dose large enough to destroy 50% of the host cells or organisms. In a similar sense, the infectious dose (ID50) is the dose which, when given to a number of test systems or hosts, causes an infection of 50% of the systems or hosts under the conditions employed. VIRUS PURIFICATION Purification makes use of several virus properties. Virions are very large relative to proteins, are often more stable than normal cell components, and have surface proteins. Because of these characteristics, many techniques useful for the isolation of proteins and organelles can be employed in virus isolation. Four of the most widely used approaches are (1) differential and density gradient centrifugation, (2) precipitation of viruses, (3) denaturation of contaminants, and (4) enzymatic digestion of cell constituents. 1. Host cells in later stages of infection that contain mature virions are used as the source of material. Infected cells are first disrupted in a buffer to produce an aqueous suspension or homogenate consisting of cell components and viruses. Viruses can then be isolated by differential centrifugation, the centrifugation of a suspension at various speeds to separate particles of different sizes . Usually the homogenate is first centrifuged at high speed to sediment viruses and other large cellular particles, and the supernatant, which contains the homogenate’s soluble molecules, is discarded. The pellet is next resuspended and centrifuged at a low speed to remove substances heavier than viruses. Higher speed centrifugation then sediments the viruses. This process may be repeated to purify the virus particles further. Viruses also can be purified based on their size and density by use of gradient centrifugation . A sucrose solution is poured into a centrifuge tube so that its concentration smoothly and linearly increases between the top and the bottom of the tube. The virus preparation, often after purification by differential centrifugation, is layered on top of the gradient and centrifuged. The particles settle under centrifugal force until they come to rest at the level where the gradient’s density equals theirs (isopycnic gradient centrifugation). Viruses can be separated from other particles only slightly different in density. Gradients also can separate viruses based on differences in their sedimentation rate (rate zonal gradient centrifugation. When this is done, particles are separated on the basis of both size and density; usually the largest virus will move most rapidly down the gradient. It that viruses differ from one 15 another and cell components with respect to either density (grams per milliliter) or sedimentation coefficient(s). Thus these two types of gradient centrifugation are very effective in virus purification. 2. Viruses, like many proteins, can be purified through precipitation with concentrated ammonium sulfate. Initially, sufficient ammonium sulfate is added to raise its concentration to a level just below that which will precipitate the virus. After any precipitated contaminants are removed, more ammonium sulfate is added and the precipitated viruses are collected by centrifugation. Viruses sensitive to ammonium sulfate often are purified by precipitation with polyethylene glycol. 3. Viruses frequently are less easily denatured than many normal cell constituents. Contaminants may be denatured and precipitated with heat or a change in pH to purify viruses. Because some viruses also tolerate treatment with organic solvents like butanol and chloroform, solvent treatment can be used to both denature protein contaminants and extract any lipids in the preparation. The solvent is thoroughly mixed with the virus preparation, then allowed to stand and separate into organic and aqueous layers. The unaltered virus remains suspended in the aqueous phase while lipids dissolve in the organic phase. Substances denatured by organic solvents collect at the interface between the aqueous and organic phases. 4. Cellular proteins and nucleic acids can be removed from many virus preparations through enzymatic degradation because viruses usually are more resistant to attack by nucleases and proteases than are free nucleic acids and proteins. For example, ribonuclease and trypsin often degrade cellular ribonucleic acids and proteins while leaving virions unaltered. ************** Pass word: virus