* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 7 Non- potentiometric methods of analysis
Survey
Document related concepts
Transcript
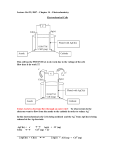
Lecture 7 Non- potentiometric methods of analysis Dr. Rasha Hanafi Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 1 Learning outcomes By the end of this session, the student should be able to: 1.Identify fundamentals of electrolysis. 2.Determine voltage changes when current flows. 3.Estimate overvoltage due to different types of polarization. 4. Describe controlled potential electrolysis with three electrode system. 5. Use electrogravimetric measurement for quantitative analysis. 6. Use coulometry and coulometric titrations for quantitative analysis Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 2 I. Potentiometric versus non-potentiometric methods of analysis Galvanic cell Dr. Rasha Hanafi, GUC Electrolytic cell PHCM662, Lecture 7, SS2016 3 I. Potentiometric vs. non-potentiometric methods of analysis Potentiometric methods 1. No external work (potential) is applied. 2. Spontaneous chemical reaction. 3. The two half-cells are set up in different containers, being connected through the salt bridge 4. No current passes due to the presence of a high resistance in the potentiometer, hence no change in conc. of analytes happen, so potentiometry is a nondestructive method of analysis. 5. By convention, left position represents always the anode. the anode is negative and cathode is the positive electrode 6. The thermodynamic potential Ecell is calculated from Nernst equation to be used in quantitative calculations. Dr. Rasha Hanafi, GUC Non- potentiometric methods 1. 2. 3. 4. 5. 6. 7. External work is applied: an external battery is needed. Current is measured by an ammeter. The chemical reaction is not spontaneous and is forced to occur. Both the electrodes are placed in a same container in the solution of molten electrolyte Consumption of analytes happen (analysis is destructive). The moles of e- flowing through the cell are “It/F” (I:current, t:time and F: Faraday constant 96485 C/mol). Positions are not important : connections of the poles of the battery control which will be the anode. the anode is positive and cathode is the negative electrode The potential of the cell can not be simply calculated from Nernst equation: current flow affects the potential measured. PHCM662, Lecture 7, SS2016 4 II. Fundamentals of electrolysis Suppose we dip Cu and Pt electrodes into an aqueous solution of Cu2+ and pass electric current through to deposit Cu2+ as Cu metal at the cathode which necessarily liberates O2 at the anode. cathode Cu 2 2e Cu ( s) Anode H 2O 12 O2 ( g ) 2 H 2e Net reaction H 2O Cu 2 Cu ( s) 12 O2 ( g ) 2 H The electrode at which the reaction of interest occurs is called the working electrode (Cu-electrode), while the other electrode is called the counter/ auxiliary electrode. Dr. Rasha Hanafi, GUC cathode PHCM662, Lecture 7, SS2016 anode CuSO4(aq) Two-electrodes cell 5 II. Fundamentals of electrolysis, cont. • Note that a negative potential is applied to the cathode where reduction occurs and a positive potential is applied to the anode where oxidation occurs. • For the aforementioned cell: Ecell = Ecathode Eanode If the cell contains 0.2 M Cu2+ and 1.0 M H+ and liberates O2 at a pressure of 1.0 bar, the thermodynamic or equilibrium cell potential will be E= 0.911 V (calculated using Nernst equation for the cell reaction as written). Thus the reaction has a negative potential which means that the reaction is not spontaneous. • If we apply a voltage slightly greater than 0.911 V between the electrodes, we will provide just enough free energy to force the reaction. • If higher current is needed (higher reaction rate), an extra voltage (overvoltage) is needed (Faraday 1st law of electrolysis: The mass of a substance altered at an electrode during electrolysis is directly proportional to the quantity of electricity transferred at that electrode. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 6 III. Voltage Changes when current flows When current passes (at a fixed applied potential, battery of fixed voltage) through an electrochemical cell, the measured cell potential departs from that derived from thermodynamic calculations (using Nernst equation)!! This is due to phenomena such as 1. Ohmic resistance. 2. Polarization effects : A. Concentration polarization . B. Charge-transfer polarization. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 7 III. 1. Ohmic resistance It is the potential needed to overcome the resistance of the ions to move toward the anode and the cathode i.e., to overcome the electric resistance (R) of the solution in the electrochemical cell when current is flowing. Eohmic = I R The resistance, R, depends on the kinds and concentrations of ions in solution Ecell = Ecathode Eanode I R, if no current passes I=0 IR=0 Thus, we have to increase the potential to operate an electrolytic cell (make it more powerful = more negative). Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 8 III. 2. Sources of polarization For the half-cell shown in figure, the overall electrode reaction is: Ox + ne Bulk Ex: Cu2+ + 2e Red Bulk For current to pass continuously across the surface of the electrode, Ox in the bulk of solution should diffuse to the electrode region in what is known as mass transfer step followed by electron transfer step when Ox reaches the electrode surface. The electrochemical reaction is completed when the formed Red diffuses back to the bulk of solution. If one of these steps is slow, it will limit the overall rate of reaction and thus reduces the magnitude of current. Dr. Rasha Hanafi, GUC Cu Steps of electrochemical reaction Electrode surface Mass transfer Ox ne Solution bulk Ox Electron transfer Red PHCM662, Lecture 7, SS2016 Red Mass transfer 9 III. 2. A. Concentration polarization It arises when the transport of reactive species to the electrode surface is insufficient (slow mass transfer) to maintain the current needed by the equation: Ecell = Ecathode Eanode I R It is observed when concentrations of the electroactive species are not the same at the surface of the electrode as in the bulk solution In case of Cu2+: Ecathode 0.34 0.0591 log [Cu 2 ]surf . 2 where [Cu2+]surf. is the concentration of Cu2+ at the electrode surface If the reduction of Cu2+ occurs more rapidly (fast electron transfer) than the diffusion of Cu2+ ions from bulk to the electrode surface (slow mass transfer), then, [Cu2+]surf. will decrease: A concentration polarization is observed. Consequently, a concentration overvoltage, c, should be applied in order to increase the rate of mass transfer from the bulk of solution to the electrode surface. Ecell = Ecathode Eanode I R c Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 10 III. 2. A. Concentration polarization, cont. In order to know how concentration polarization can be prevented or induced as required, it is important to investigate the mechanisms by which ions are transported from the bulk of solution to the electrode surface. An electroactive species has three ways to reach the surface of an electrode: 1. Diffusion through a concentration gradient. Whenever a concentration difference develops between two regions of solution, as it does when a species is reduced at a cathode surface (or oxidized at an anode surface), ions move from the more concentrated region to the more dilute as a result of diffusion. 2. Migration, the process by which ions move under the effect of an electrostatic field where they are attracted or repelled by a charged surface. 3. Convection, which is the movement of bulk fluid by mechanical means such as stirring or agitation. Concentration polarization is observed when diffusion, migration and convection are insufficient to transport the reactant to or from electrode surface at a rate demanded by the theoretical current. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 11 III. 2. B. Activation or charge transfer polarization It arises when the rate of the oxidation or reduction reaction at one or both electrodes is not rapid enough (slow charge transfer) to yield currents demanded by the theory. After application of external potential So, an activation overvoltage, a, is needed to activate the reactant to pass across the electrode surface and to facilitate the charge transfer step. To sum up, Ohmic resistance, concentration and activation polarization make electrolysis more difficult. They make the cell potential more negative (decrease the potential of galvanic cell). Ecell = Ecathode Eanode IR c a As a result more voltage (overvoltage) is needed from the power supply to drive the reaction of the cell forward. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 12 III. 3. Controlled potential electrolysis with 3-electrode cell • In general we need to adjust the potential of the working electrode so that some electroactive species react while others do not. • Metal working electrodes are generally polarizable, which means that their potentials easily change when small current flow. • On the other hand, a reference electrode is said to be non-polarizable, because its potential does not vary with the flow of current. Thus, in order to measure and control the potential of a polarizable working electrode, a third reference electrode should be introduced The working electrode is the one at which the reaction of interest occurs The reference electrode is used to measure the potential of the working electrode The auxiliary electrode (the counter electrode) is the current supporting partner of the working electrode. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 13 III. 3. Controlled potential electrolysis with 3-electrode cell, cont. • In three electrode configuration, a potential is applied between the auxiliary and working electrodes and the potential of only the working electrode (measured with respect to the reference electrode) is monitored. • In controlled potential electrolysis, The voltage difference between the working electrode and reference electrode in a three-electrode cell is adjusted by an electronic device called potentiostat. •If the applied potential is set at E1, the respective potential on the working electrode will be sufficient to deposit only Cd2+ ions via reduction to Cd. Current Example: Analysis of Cd2+ and Pb2+ mixture •If the potential is adjusted at more negative value of E2, both cations will be deposited simultaneously. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 Pb2+ Cd2+ Pb Cd E1 E2 -ve potential 14 III. 3. Control of the applied voltage Observed current-voltage relationship for • Nothing special happens at -0.911 V electrolysis of 0.2 M CuSO4 solution (voltage calculated from Nernst). At this low voltage, a small residual current is observed as a result of reduction of traces of dissolved O2 or some Fe3+ impurities. • Near 2.0 V, electrolysis of Cu2+ starts and the rate of reaction (the reduction current) increases steadily. Cu2+(aq) + 2e- Cu(s) • The voltage between the two electrodes is E = Ecathode Eanode IR overpotential Suppose we hold the applied voltage at E = 2.0 V until all Cu2+ is reduced . As Cu2+ is used up (at the end of electrolysis), the current decreases and both the ohmic and overpotentials decrease. Note that During Eanode is fairly constant because of the high concentration of solvent (H2O) being oxidized at the anode. Since the applied voltage was held End constant, Ecathode eventually becomes more negative in order to keep the integral equality in the above equation. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 -2 = x -1 -0.5 ; x=-0.5 -2 = x -1 -0 ; x= -1!! 15 III. 3. Control of the applied voltage, cont. • When the applied potential is held constant, the potential of the cathode may become negative enough that unintended reductions may occur: 1. reduction of H+ or water to H2 gas. The gas bubbles evolved at the cathode surface interfere with the deposition of the solid. 2. reduction of other ions such as Co2+, Sn2+ or Ni2+. • To prevent the cathodic evolution of H2 gas at the cathode, a cathodic depolarizer such as NO3- can be added to the solution. It is more easily reduced than H+ or any other interfering species. NO3 10H 8e NH 4 3H 2 O • Alternatively, we can use three-electrode cell with potentiostat to control the cathodic potential and prevent unwanted side reactions. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 16 Electroanalytical methods may be based on the measurement of either: 1. Current at a fixed potential. 2. Potential at a fixed current. Today’ s lecture Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 17 IV. Electrogavimetric Analysis • The analyte is quantitatively deposited on an electrode by electrolysis. • The electrode is weighed before and after deposition, the increase in mass quantifies the analyte. Cu2+ in solution can be quantified by reducing it to Cu(s) on a carefully cleaned Pt gauze cathode with a large surface area. O2 is liberated at the counter electrode (decomposition of water). How do you know electrolysis is complete? 1. disappearance of color in solution (if the analyte solution is colored). 2. to take one drop of solution and perform a qualitative test for analyte confirming its total absence. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 18 V. Coulometric titrations Coulometric titration (const. current): 2Br Br2 2e (Generation of Br2 titrant) form of quantitative analysis based on counting the number of moles of electrons used in a reaction. Ex: cyclohexene (CYC) can be titrated with Br2 generated by electrolytic anodic oxidation of Br-. The initial solution contains an unknown quantity of CYC and a large amount of Br-. Generation circuit Detection circuit The reaction is carried out at constant current. Br2 generated at the Pt generator anode immediately reacts with CYC. When CYC is consumed, the concentration of Br2 suddenly rises, signaling the end of the titration. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 19 Coulometric titration (const. current), cont.: The rise in Br2 concentration is detected by measuring the current between two Pt detector electrodes (in the detection circuit). A voltage of 0.25 V applied between these two electrodes is not enough to electrolyze any solute, so only a tiny current of <1 A flows through the microammeter. At the equivalence point, CYC is consumed, [Br2] suddenly increases and the detector current flows as a result of the reaction: - Detector cathode: Br2 + 2e Detector anode: 2Br- 2Br Br2 + 2e- - Note that both Br2 and Br must be present for the detector half-reactions to occur. In coulometric titration, the time needed to generate (at constant current) equivalent amount of Br2 to the analyte is measured. Applications and Advantages of coulometry 1. Fully automated coulometers commonly generate H+, OH-, Ag+ and I2 to titrate a variety of analytes including CO2, sulfides in food and sea water as well as H2O in proteins and purified solvents. 2. Unstable titrants that can not be stored or standardized such as Ag2+, Cu+, Mn3+ and Ti3+ can be generated and used immediately in titration. 3. Toxic titrants (Br2) that can not be used safely in conventional titration methods can be generated and consumed as soon they are formed in coulometric vessel. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 20 V. How to quantify in coulometry Faraday’s law: The amount of chemical reaction at an electrode (e.g., mass of copper deposited on the cathode surface) is proportional to the quantity of electricity passed in the circuit. Faradaic current: Current that passes in the circuit as a result of actual electrolysis (oxidation and reduction at electrode surface). q I . t Coulombs Amperes Moles of e Also, q= n . F seconds ( n) I .t F The coulomb is the quantity of charge that is transported in 1 sec. by a constant current of 1 A. If a reaction requires n electrons per mole of reactant, the quantity reacting of chemical species in time t is I . t Moles of substances reacted Mass Dr. Rasha Hanafi, GUC n F I .t ( molar mass) n F PHCM662, Lecture 7, SS2016 Faraday’s Law 21 References 1. “Principles of instrumental analysis, 5th ed. by Skoog, Holler, Nieman” Chapter 22 and Chapter 24. 2. “Quantitative Chemical Analysis, 6th ed. Daniel Harris” Chapter 17. 3. Lecture of “Non- potentiometric methods of analysis ” by Dr. Raafat Aly, GUC, spring 2010. Dr. Rasha Hanafi, GUC PHCM662, Lecture 7, SS2016 22