* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download graphingtrendschemistry
Survey
Document related concepts
Transcript
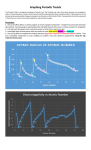
Atomic Mass vs Atomic Number 300 Potassium 250 Sodium 200 Atomic Mass Lithium 150 Bromine Chlorine 100 Fluorine Krypton Argon 50 Neon Helium 0 0 5 10 15 20 25 30 35 40 Atomic Number Ionization Energy vs Atomic Number Helium 2500 Neon 2000 Ionization Energy Fluorine Argon 1500 Krypton Chlorine Bromine 1000 500 Lithium Sodium Potassium 0 0 5 10 15 20 Atomic Number 25 30 35 40 Electronegativity vs Atomic Number 4.5 Fluorine Electronegativity (Pauling Scale) 4 3.5 Chlorine Bromine 3 2.5 2 1.5 Lithium Potassium 1 Sodium 0.5 Helium Argon Neon Krypton 0 0 5 10 15 20 Atomic Number 25 30 35 40 ANALYSIS QUESTIONS: 1. Based on your graphs, what is the trend in atomic radius across a period? Down a family? The noble gas family elements have the lowest atomic radii. The halogens family elements have the second-lowest atomic radii. The alkali metal family elements have the highest atomic radii. The farther to the left of a period an element is, the higher its atomic radius will be. 2. Based on your graphs, what is the trend in ionization energy across a period? Down a family? The alkali family elements have the lowest ionization. The halogen family elements have the second-highest ionization, and the noble gas family elements have the highest ionization. The farther to the left of a period an element is, the lower the ionization will be. 3. Based on your graphs, what is the trend in electronegativity across a period? Down a family? The noble gas family has the lowest electronegativity. The alkali family elements have the second highest electronegativity, and the halogen family elements the highest. The electronegativity is lowest towards both ends of a period, and higher in the middle. 4a) What is happening to the number of protons and the number of energy levels as you move across the periodic table from left to right? How and why does this affect atomic radius. As you move across the periodic table, the atomic radius decreases. Electrons are being added to the same energy level, but at the same time, protons are being added to the nucleus. This creates a higher effective nuclear charge, which means that the force of attraction pulling the electrons to the nucleus is stronger, resulting in a smaller atomic radius. b) What happens to the number of energy levels as you move down a column on the periodic table. How and why does this effect ionization energy? As you move down the periodic table, ionization energy decreases. Electrons become further from the nucleus which makes it easier to remove the outermost one. The inner electrons in elements with lower energy levels block the proton’s force of attraction toward the nucleus, so it’s easier to remove the outer electrons. c) What happens to the effective nuclear charge as you move across a period on the periodic table? How does this effect ionization energy and electronegativity? As you move across the period table (left to right) the nuclear charge increases. With increased nuclear charge, you also get increased attraction that the atom has for electrons in it’s outer shell, which means increased electronegativity. Ionization energy will decrease because the attraction to the nucleus will decrease. 5a) Which group contains elements which are easiest to ionize? Explain why this is the case. The alkali family is the easiest to ionize because these elements have the least amount of ionization energy. That means their valence electrons can be taken easily. The atoms of these elements are relatively large and have their outer electrons farther away from the nucleus, making it easier for them to lose electrons and form positive ions. b) Explain why the third ionization energy of Ca would be much higher than the 1st and 2nd ionization energy Ca. The third ionization energy of Ca would be much higher than the first and second because it becomes progressively more difficult to remove electrons. An atom of calcium will be less willing to let go of its third valence electron that its first and second. Removing a negative electron from a positive calcium ion is more difficult that removing a negative electron from a regular calcium atom. 6. Which element would have the highest electronegativity in each set below? Explain why this is. a) Ca, Be or Mg b) B, Li, or F a.) Beryllium would have the highest electronegativity because it’s smaller than the other two, and therefore its nucleus is closer to the surface and can take electrons far more easily than the other two elements. b.) Fluorine would have the highest electronegativity in this set, and it’s actually the element with the highest electronegativity out of all the elements, and in general, elements in the halogen element group have high electronegative values. 7. Write (or type) the electron configuration of each atom (high-light the valence electrons) and its corresponding ion below each sketch (atomic radii are given in angstroms (1 x 10 -10 M). 8. Over the blank periodic table provided, write or type the number of valence electrons and the expected ion charge for the transition metal block and for the families to the left and right of the transitions metals (the alkali metals have been done as an example). Note: Carbon and boron do not normally form ions and are thus blanked-out