* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Lateral Tunnel Fontan Procedure for Hypoplastic Left Heart
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
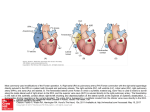
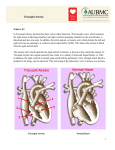
Pediatr Cardiol (2007) 28:426–432 DOI 10.1007/s00246-007-9002-5 The Lateral Tunnel Fontan Procedure for Hypoplastic Left Heart Syndrome: Results of 100 Consecutive Patients J. C. Hirsch Æ R. G. Ohye Æ E. J. Devaney Æ C. S. Goldberg Æ E. L. Bove Published online: 3 August 2007 Ó Springer Science+Business Media, LLC 2007 Abstract The Fontan procedure for hypoplastic left heart syndrome (HLHS) is well established. Multiple surgical techniques including extracardiac conduits and autologous tissue connections have been developed. We reviewed the results of 100 consecutive patients undergoing the lateral tunnel modification of the Fontan procedure at the University of Michigan. A cross-sectional retrospective study was performed for 100 consecutive patients identified in the University of Michigan Congenital Heart Surgery database with the diagnosis of HLHS. All patients had undergone a lateral tunnel Fontan procedure between June 2000 and August 2004. The medical record was reviewed to assess patient, procedural, and morphologic determinants of outcome. Hospital survival was 97% and intermediate-term survival was 96% with a median follow-up time of 34 months. Preoperative mean pulmonary artery pressure, right ventricular end diastolic pressure, aortic cross-clamp time, and tricuspid valve regurgitation were not associated with late right ventricular function or survival. Three patients required takedown of the lateral tunnel Fontan in the early postoperative period. A positive association was found between protein-losing enteropathy and prolonged (>2 weeks) postoperative pleural drainage (p = 0.035). No patient required cardiac transplantation or J. C. Hirsch (&) R. G. Ohye E. J. Devaney E. L. Bove Section of Cardiac Surgery, Division of Pediatric Cardiovascular Surgery, University of Michigan School of Medicine, 1500 E. Medical Center Drive, Mott F7380, Ann Arbor MI 48109-0223, USA e-mail: [email protected] C. S. Goldberg Section of Pediatric Cardiology, University of Michigan School of Medicine, 1500 E. Medical Center Drive, Mott F7380, Ann Arbor, MI 48109-0223, USA 123 late intervention on the Fontan pathway. At the time of follow-up, 100% of patients were New York Heart Association class I or II and 90% were in normal sinus rhythm. The lateral tunnel Fontan procedure for HLHS can be performed with acceptable early and intermediate-term risk. There was a low prevalence of late rhythm disturbances and other complications. Protein-losing enteropathy and prolonged pleural drainage were associated. Staged palliation for the surgical management of single ventricle congenital heart defects is widely accepted, but with variable techniques employed at each individual stage [2, 5]. However, the overall benefits and risks of these modifications have not been rigorously tested. The choice of technique is often based on surgeon/center preference. At the University of Michigan, more than 600 Fontan procedures have been performed between 1992 and 2005. For patients with suitable anatomy, we continue to prefer the lateral tunnel technique. Our choice of the lateral tunnel Fontan is partially based on our preference for the hemi-Fontan over the bidirectional Glenn as the second-stage palliation. The hemiFontan creates a large cavopulmonary connection that facilitates augmentation of the branch pulmonary arteries and simplifies the completion of the inferior vena caval pathway at the time of the Fontan (Fig. 1). Although the hemi-Fontan is a technically more difficult procedure, the benefits are worth the increased effort at the hemi-Fontan stage when a longer operation is better tolerated in the postoperative phase than following the more hemodynamically demanding recovery period after the Fontan. In a prior publication from our institution, this method of staged Pediatr Cardiol (2007) 28:426–432 427 Fig. 1 The anastomosis between the cavoatrial junction and the central pulmonary arteries is shown on the left. The final reconstruction allows only superior vena cava return to enter the lungs while retaining the connection with the right atrium to simplify the subsequent lateral tunnel Fontan operation. Reprinted with permission from Bove and Mosca [5] Fig. 2 Three-dimensional model of typical hemi-Fontan procedure based on anatomic data from MR scans and angiocardiograms. Reprinted with permission from Bove et al. [3] repair resulted in a survival rate of 94% for patients undergoing a hemi-Fontan followed by a lateral tunnel Fontan [8]. In addition, computational fluid dynamic modeling of cavopulmonary connections for hypoplastic left heart syndrome (HLHS) has demonstrated the optimal caval offset achieved by the lateral tunnel Fontan with the lowest power loss compared to traditional total cavopulmonary connections. The energy-efficient flow within the cavopulmonary connection of the hemi-Fontan (Fig. 2) followed by the lateral tunnel Fontan (Fig. 3) procedure is due to the streamlined geometry of the anastomotic design. This study demonstrated the important hemodynamic differences between the hemi-Fontan and bidirectional Glenn, which only become apparent following completion of the Fontan procedure. The ‘‘optimal’’ lateral offset of the bidirectional Glenn combined with the lateral tunnel or extracardiac Fontan directs the superior vena cava (SVC) flow preferentially into the right pulmonary artery, whereas the larger volume inferior vena Fig. 3 Model of completion lateral tunnel Fontan as performed after hemi-Fontan procedure by removal of the intraatrial patch. Reprinted with permission from Bove et al. [3] cava (IVC) flow is directed to the smaller left lung. In contrast, the blood flow into the branch pulmonary arteries is not only more efficient with less energy losses but also more uniformly distributed to each lung with the hemi-Fontan/lateral tunnel Fontan approach [3]. This increased performance appears to be the result of the divergent shape in both the SVC and the IVC anastomoses and the anteroposterior offset of the two caval anastomoses in relation to the pulmonary arteries [2]. Since 1992, 94% of single ventricle patients at the University of Michigan have received a lateral tunnel Fontan as the third-stage palliation. To improve our understanding of the intermediate-term outcomes for the lateral tunnel Fontan, we performed this retrospective study 123 428 Pediatr Cardiol (2007) 28:426–432 Fig. 4 A polytetrafluoroethylene patch is fashioned to channel inferior vena caval return to the previously constructed anastomosis with the pulmonary arteries. The tunnel is completed by suturing the patch within the right atriotomy closure to evaluate outcomes in a single diagnostic group (HLHS) undergoing the lateral tunnel Fontan procedure at the University of Michigan. Materials and Methods Data Collection We performed a cross-sectional retrospective study on 100 consecutive patients identified through the University of Michigan Congenital Heart Surgery database with HLHS and a lateral tunnel Fontan procedure performed between June 2000 and August 2004. Only patients with classic HLHS were included, as defined by right ventricle-dependent systemic circulation and systemic outflow tract obstruction. We obtained preoperative, operative, and hospital course data on each patient through a review of paper and electronic medical records. Preoperative data collected were patient demographics, cardiac catheterization information on both mean pulmonary artery (PA) pressures and right ventricular end diastolic pressure (RVEDP), and echocardiographic results of right ventricular function and tricuspid valve regurgitation. We converted right ventricular function and tricuspid valve regurgitation to ordinal variables. For right ventricular function, 0 = normal, 1 = mildly depressed, 2 = moderately depressed, and 3 = severely depressed. For tricuspid valve regurgitation, 0 = none or trace, 1 = mild, 2 = moderate, and 3 = severe. Operative data included age and weight at repair, cardiopulmonary bypass (CPB) and aortic crossclamp (XC) times, and concurrent procedures. Hospital course data encompassed postoperative arrhythmias, chest tube drainage >2 weeks, additional procedures including takedown of the Fontan, discharge right ventricular function 123 and tricuspid valve regurgitation based on echocardiographic data, discharge rhythm, and length of stay. Follow-up data obtained from our pediatric cardiology clinic notes or from communication with the referring cardiologist were length of follow-up, the presence of a fenestration, New York Heart Association (NYHA) classification, most recent echocardiographic data on right ventricular function and tricuspid valve regurgitation, current rhythm, interval interventions or operations, current medications, and any subsequent complications. Institutional review board approval was obtained prior to the initiation of the study. Surgical Technique All 100 patients had undergone a prior hemi-Fontan procedure according to techniques described previously [2]. The lateral tunnel procedure was performed during continuous cardiopulmonary bypass at 28–32°C. The IVC pathway was constructed with a patch of 0.6-mm Gore-Tex (W. L. Gore & Associates, Flagstaff, AZ, USA). The patch was sutured around the internal orifice of the IVC and continued above the right pulmonary veins until the previous patch placed at the hemi-Fontan procedure was encountered. The previous patch was excised in its entirety. A 2.8- or 3-mm punch was utilized to construct a fenestration. The Fontan was completed by closing the atriotomy incorporating the anterior half of the Gore-Tex patch (Fig. 4) [6]. Mediastinal and bilateral pleural drains were placed in the operating room in all patients. Statistical Analysis Data were recorded on a Microsoft Excel spreadsheet (Microsoft, Redmond, WA, USA) and transferred to the Pediatr Cardiol (2007) 28:426–432 429 Table 1 Patient demographic, preoperative, and intraoperative data Table 2 Concomitant surgical procedures Pre- and intraoperative parametric data, mean ± SD Procedure n Age (months) 24 ± 6 Tricuspid valve repair 15 Weight (kg) 11.1 ± 1.5 Pulmonary artery augmentation 2 Mean PA pressure (mmHg) 13 ± 3 Epicardial pacemaker placementa 2 RVEDP (mmHg) 8±3 a CPB time (min) 65 ± 19 XC time (min) 28 ± 9 One for preoperative complete heart block and one for preoperative sick sinus syndrome Preoperative nonparametric data (n = 100), % Table 3 Postoperative morbidity RV systolic function Normal 79 Mild dysfunction 18 Moderate dysfunction Severe dysfunction 3 0 Tricuspid valve regurgitation None/trace 41 Mild 37 Moderate 16 Severe 6 Complication No. (n = 100) Postoperative arrhythmia 19 Prolonged pleural drainage (>2 weeks) 16 Seizure 5 Diaphragm plication 4 Thoracic duct ligation 4 Fontan takedown 3 Stroke 2 Epicardial pacemaker placement 2 CPB, cardiopulmonary bypass; PA, pulmonary artery; RV, right ventricle; RVEDP, right ventricular end diastolic pressure; SD, standard deviation; XC, cross-clamp ECMO 1 SAS statistical package (SAS Institute, Cary, NC, USA) for analysis. Normal data are expressed as mean ± standard deviation and non-normal data as medians and ranges. Differences in dichotomous variables were compared by using chi-square or Fisher’s exact test. Wilcoxon rank sum tests were performed for comparisons involving ordinal variables. concomitant procedure (n = 15). When patients were subcategorized into those who did and those who did not receive a tricuspid valve repair, we observed no significant difference in tricuspid valve regurgitation at the time of discharge or follow-up, nor was there a significant difference in long-term RV function or survival. ECMO, extracorporeal membrane oxygenation Postoperative Data Results Preoperative and Intraoperative Data A summary of the preoperative and intraoperative data is presented in Table 1. We defined a high RVEDP as >9 mmHg and high PA pressure as 15 mmHg representing the upper quartiles. Longer XC time was defined as any time greater than the median of 25 minutes. High RVEDP and high PA pressure did not correlate with late RV function. Longer XC times demonstrated a trend toward decreased late RV function (p = 0.07), but this was not statistically significant. There was also a trend toward lower preoperative PA pressure ( 15 mmHg) having an association with postoperative survival (p = 0.054). Preoperative RV function did not predict postoperative arrhythmias. Table 2 summarizes the operative procedures performed in combination with the lateral tunnel Fontan procedure. Tricuspid valve repair was the most common The postoperative complications are listed in Table 3. Major complications include arrhythmias, prolonged pleural drainage, and seizures. Table 4 contains information on RV function, tricuspid valve regurgitation, and rhythm both at the time of discharge and at follow-up. Although 19% of patients had an arrhythmia postoperatively (complete heart block, junctional rhythm, junctional ectopic tachycardia, atrial flutter, supraventricular tachycardia, and sick sinus syndrome), 95% of patients were discharged home in normal sinus rhythm. Two patients required placement of a permanent pacemaker in the postoperative period. One of these patients developed complete heart block following a tricuspid valvuloplasty and the other patient had sick sinus syndrome, which was poorly tolerated. We dichotomized the length of time for pleural drainage into patients draining for <2 weeks or >2 weeks. Sixteen percent of patients drained for >2 weeks. Prolonged chest tube drainage was significantly associated 123 430 Pediatr Cardiol (2007) 28:426–432 Table 4 Discharge and follow-up data Table 5 Late complications and interventions Discharge (%) Complication No. (n = 81) Follow-up (%) PLE RV systolic function 5 Normal 70 84 Late death 4 Mild dysfunction 28 14 Device fenestration closure 3 Moderate dysfunction 2 2 Arrhythmia 2 Severe dysfunction 0 0 Fenestration creation 1 Tricuspid valve regurgitation Recurrent effusions 1 None/trace 48 52 Stroke 1 Mild 37 35 Seizure 1 Moderate 15 13 Tricuspid valve replacement 1 0 0 Severe Rhythm Normal sinus rhythm 95 90 Junctional 2 3 Sick sinus syndrome 0 5 Paced 3 3 RV, right ventricle with the development of protein-losing enteropathy (PLE) (p = 0.035). Prolonged chest tube drainage and PLE had no association with the late presence of a fenestration. Three patients required takedown to a hemi-Fontan in the postoperative period. The median length of stay in the hospital was 10 days (range, 3–121). The overall hospital survival was 97%. One patient developed refractory supraventricular tachycardia requiring emergent placement on extracorporeal membrane oxygenation for support. This patient had a significant anoxic brain injury and the family chose to withdraw support. Another patient with sepsis and low cardiac output developed persistent seizure activity and progressed to brain death. The third patient underwent multiple interventions on the left pulmonary artery including a stent. Following the Fontan, the patient’s left pulmonary artery thrombosed and the patient was converted to a hemi-Fontan but still progressed to multisystem organ failure and support was withdrawn. Follow-Up Data Of the 100 patients who underwent a lateral tunnel Fontan procedure during the study period, 84% of the hospital survivors were available for follow-up. The median followup time was 34 months (range, 1–72). All patients were in NYHA class I or II. Table 4 contains information regarding RV function, tricuspid valve regurgitation, and rhythm at the time of follow-up. Table 5 outlines late complications in patients available for follow-up. We could confirm the status of the fenestration in 55 patients and found patency 123 PLE, protein-losing enteropathy in 62%. One patient required creation of a fenestration for high Fontan pathway pressure and PLE. Three patients underwent fenestration closure with an Amplatzer device (AGA Medical, Plymouth, MN, USA). All of the patients are taking aspirin 40.5 mg per day except for 4 patients who were on coumadin. The indications for coumadin included left pulmonary artery stent, mechanical tricuspid valve, superior vena caval thrombus, and left ventricle thrombus. We noted 4 late deaths between 2 and 46 months following the lateral tunnel Fontan procedure. No patients required transplantation, Fontan conversion, or intervention on their Fontan pathway. Discussion Surgical and medical management of patients with a functional single ventricle has been significantly refined since 1971, when Fontan and Baudet first described total cavopulmonary anastomosis for the palliation of tricuspid atresia [11]. After de Leval et al. as well as Jonas and Castaneda introduced the lateral tunnel Fontan procedure, the longterm survival rate for patients with HLHS improved, as did their freedom from late arrhythmias [7, 13, 19]. This study demonstrates excellent hospital and intermediate-term survival in the highest risk single ventricle population. A number of studies have demonstrated that elevated pulmonary artery pressures and resistance, pulmonary artery distortion, impaired ventricular function, significant tricuspid valve regurgitation, and prolonged cardiopulmonary bypass and aortic cross-clamp times adversely influence survival [4, 14, 15]. Recent improvements in operative management have neutralized many of these risk factors. In a prior report from our group, survival was adversely affected by elevated preoperative PA pressures and prolonged CPB times [16]. In this study, which examined a similar patient population, decreased survival was associated with PA pressures 15 mmHg (p = 0.054), but this Pediatr Cardiol (2007) 28:426–432 trend did not reach statistical significance. In the current study, we were unable to demonstrate an association between CPB and XC time with survival or late RV function. These findings may be attributable to modifications in our surgical technique demonstrated in an earlier study [4]. Given the small sample size and overall excellent survival and functional status, our study may not have adequate power to determine these relationships. We previously demonstrated the importance of concomitant tricuspid valve repair for improved survival and preserved RV function in patients with significant tricuspid valve regurgitation [18]. This study again demonstrated that tricuspid valvuloplasty could produce successful and durable outcomes. We continue to incorporate tricuspid valvuloplasty as part of the lateral tunnel Fontan procedure when warranted; 15% of patients underwent a concomitant repair in this study. Improvements in tricuspid valve regurgitation and RV function at the time of discharge and late follow-up were durable in this population. Furthermore, the addition of the valvuloplasty, despite longer cardiopulmonary bypass times, did not negatively affect survival. As the operative success for single ventricle patients improves and the survivors of the procedure age, concern regarding late complications has increased. Late risks include arrhythmias and PLE. Early postoperative arrhythmias, atriopulmonary connections, and significant atrioventricular valve regurgitation have been previously shown to be associated with late arrhythmias [1, 9]. In our cohort of patients, none of these could be shown to be associated with late arrhythmias. However, 90% of our patients had a normal sinus rhythm at the time of followup, and only two patients had late dysrrhythmias. Proponents of extracardiac conduit Fontan procedures believe there may be a decreased incidence of atrial arrhythmias with this technique due to fewer atrial suture lines and the avoidance of exposure of the atrium to elevated pressures [17]. Although this was not demonstrated in the current study, follow-up remains short. The pathogenesis of PLE remains poorly understood. Feldt et al. [10] found multiple factors associated with the late development of PLE in Fontan patients, including ventricular anatomy other than dominant left ventricle, elevated preoperative end diastolic pressure, prolonged CPB time, and lengthened hospital stay (>15 days). These authors did not specifically evaluate prolonged postoperative pleural effusions, but they speculated that there might be an association. In this study, we demonstrated an association between PLE and pleural drainage >2 weeks. However, no association was found between PLE and high RVEDP or prolonged CPB time. This may be due to the relatively short CPB times in this study. Furthermore, the moderate length of follow-up in this study may not be adequate to fully evaluate the risk of developing PLE. 431 In conclusion, we continue to prefer the lateral tunnel Fontan for the third-stage palliation in functional single ventricle patients with suitable anatomy. Based on computational fluid dynamic modeling, the hemi-Fontan procedure followed by the lateral tunnel Fontan procedure minimizes energy losses and optimizes the distribution of IVC blood flow into both lungs [3]. In a cohort of patients from our institution, neurodevelopmental outcomes for preschool and early school-age children following the Fontan operation were found not to be statistically different from the general population, even in patients with HLHS [12]. The use of autologous tissue for the majority of the reconstruction may allow for growth potential and reduce the theoretical risk of thromboembolism. With the exception of a specific indication for coumadin, the patients in this cohort were managed on low-dose aspirin alone, without any documented cases of thromboembolism. There are limitations to this study that could be addressed in future studies. The length of follow-up may be insufficient to detect the incidence of late complications of the Fontan procedure, including PLE, arrhythmias, and late thrombus formation. The results represent the experience of a single institution. Multicenter studies comparing extracardiac and lateral tunnel Fontan procedures for specific diagnoses such as HLHS will help elucidate differences between these procedures in a larger cohort and eliminate single center bias. References 1. Alphonso N, Baghai M, Sundar P, et al. (2005) Intermediateterm outcome following the Fontan operation: a survival, functional, and risk-factor analysis. Eur J Cardiothorac Surg 28:529–535 2. Bove EL (1998) Current status of staged reconstruction for hypoplastic left heart syndrome. Pediatr Cardiol 19:308–315 3. Bove EL, de Leval MR, Migliavacca F, et al. (2003) Computational fluid dynamics in the evaluation of hemodynamic performance of cavopulmonary connections after the Norwood procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 126:1040–1047 4. Bove EL, Lloyd TR (1996) Staged reconstruction for hypoplastic left heart syndrome, contemporary results. Ann Surg 224:387–394 5. Bove EL, Mosca RS (1996) Surgical repair of the hypoplastic left heart syndrome. Prog Pedeatr Cardiol 5:23–35 6. Bove EL, Ohye RG, Devaney EJ (2004) Hypoplastic left heart syndrome: conventional surgical management. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 7:3–10 7. de Leval MR, Kilner P, Gewillig M, Bull C (1988) Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg 96:682– 695 8. Douglas WI, Goldberg CS, Mosca RS, et al. (1999) Hemi-Fontan procedure for hypoplastic left heart syndrome: outcome and suitability for Fontan. Ann Thorac Surg 68:1361–1367 123 432 9. Durongpisitkul K, Porter CJ, Cetta F, et al. (1998) Predictors of early- and late-onset supraventricular tachyarrhythmias after Fontan operation. Circulation 98:1099–1107 10. Feldt RH, Driscoll DJ, Offord KP, et al. (1996) Protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg 112:672–680 11. Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26:240–248 12. Goldberg CS, Schwartz EM, Brunberg JA, et al. (2000) Neurodevelopmental outcome of patients after the Fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr 137:646–652 13. Jonas RA, Castaneda AR (1988) Modified Fontan procedure: atrial baffle and systemic venous to pulmonary artery anastomotic techniques. J Card Surg 3:91–96 14. Kaulitz R, Ziemer G, Luchmer I, et al. (1996) Modified Fontan operation in functionally univentricular hearts: preoperative risk factors and intermediate results. J Thorac Cardiovasc Surg 112:658–669 123 Pediatr Cardiol (2007) 28:426–432 15. Mayer JE Jr, Bridges N, Lock K, et al. (1992) Factors associated with marked reduction in mortality for Fontan operations in patients with single ventricle. J Thorac Cardiovasc Surg 103:444– 452 16. Mosca RS, Kulik TJ, Goldberg CS, et al. (2000) Early results of the Fontan procedure in one hundred consecutive patients with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 119:1110–1118 17. Nurnberg JN, Ovroutski S, Alexi-Meskishvili V, et al. (2004) New onset arrhythmias after the extracardiac conduit Fontan operation compared with the intra-atrial lateral tunnel procedure: early and midterm results. Ann Thorac Surg 78:1979– 1988 18. Ohye RG, Gomez CA, Goldberg CS, et al. (2004) Tricuspid valve repair in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 127:465–472 19. Stamm C, Friehs I, Mayer JE Jr, et al. (2001) Long-term results of the lateral tunnel Fontan operation. J Thorac Cardiovasc Surg 122:1219–1228