* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download U1 Periodic Trends - Alliance Ouchi-O`Donovan 6
Survey
Document related concepts
Transcript
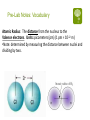
Atomic Theory and the Periodic Table Day 6 & 7 Boon Chemistry 08.19_21.13 Homework Review: p. 122 #6, 7, 11, 12 6. What do elements in the same period have in common? A: They have the same number of energy levels with electrons in them. For example, the period 2 elements have electrons in the 1st and 2nd energy levels. Homework Review: p. 122 #6, 7, 11, 12 7. What do elements in the same group have in common? A: They have the same number of valence electrons. For example, the group 1elements have 1 valence electron. Homework Review: p. 122 #6, 7, 11, 12 11. What determines the number of elements found in each period in the periodic table? A: The number of available electron orbitals in each energy level determines the number of elements in a period. Homework Review: p. 122 #6, 7, 11, 12 12. Are elements with similar chemical properties more likely to be found in the same period or in the same group? A: The same group because the number of valence electrons determine the chemical properties. For example, the alkaline earth metals all have 2 valence electrons so they form ionic bonds with Fluorine in the proportion: XF2 Periodic Trends Lab: Introductory Video As you watch, think about the following: Mendeleev put together his periodic table in the 1860s. What was known about atoms at that time? What properties did Mendeleev use to categorize and order the elements? Periodic Trends Lab: Introduction A trend is a predictable change in a certain direction. Understanding periodic trends allows us to make predictions about the chemical behavior of the elements. Example: Metallic properties of elements decrease from left to right across the table. Periodic Trends Lab: Introduction In this investigation, your lab group will recreate Dimitri Mendeleev’s discovery of the classification of the elements and the periodic law using a deck of special element cards. The real properties of the elements, but not their names or symbols, are written on these cards. As the cards are arranged and rearranged based on logical trends in some of these properties, the nature of the periodic law should reveal itself. Pre-Lab Notes: Vocabulary Atomic Radius: The distance from the nucleus to the Valence electrons. Units: picometers (pm) (1 pm = 10-12 m) •Note: determined by measuring the distance between nuclei and dividing by two. ATOMIC SIZE/RADIUS The more energy levels (bigger period number) the bigger the atom The more protons an element has the more the electrons are pulled toward the nucleus, making the atom smaller Pre-Lab Notes: Vocabulary Ionization Energy: The energy required to take An electron away from an atom. Units: kilocalories per mole (kCal/mol). Atoms that want to gain electrons have high ionization energies. Atoms that want to lose electrons have low ionization energies. Noble gases have the highest ionization energy. Pre-Lab Notes: Vocabulary Electronegativity: A measure of an atom’s tendency to attract electrons towards itself. Units: none, scale of 0-4 with Fluorine at 4 (highest). Here, Cl is more Atoms that want to gain electrons have high electronegativity. Atoms that want to lose electrons have low electronegativity. Noble gases have no electronegativity. electronegative than H. Cl pulls more electrons towards it.