* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Echocardiographic Evaluation Before Bidirectional Glenn Operation
Coronary artery disease wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Aortic stenosis wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
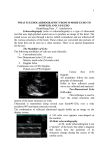
Echocardiographic Evaluation Before Bidirectional Glenn Operation in Functional Single-Ventricle Heart Disease Comparison to Catheter Angiography Kenan W.D. Stern, MD; Doff B. McElhinney, MD; Kimberlee Gauvreau, ScD; Tal Geva, MD; David W. Brown, MD Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 Background—Cardiac catheterization is routinely performed in patients with single ventricle before bidirectional Glenn operation (BDG). There is interest in noninvasive evaluation alone before BDG, but concern for echocardiography successfully imaging the relevant anatomy persists. We evaluated the accuracy of echocardiographic imaging of vascular anatomy. Methods and Results—Diagnostic images of 130 patients who had echocardiography and catheterization before BDG were reviewed; diameters of the pulmonary arteries (PAs) and aortic arch were measured, and stenoses were recorded. Patient and procedural factors associated with echocardiographic imaging were analyzed. Median age at echocardiography was 4 months; the most common diagnosis was hypoplastic left heart syndrome (55%). The left PA was imaged by echocardiography in 83 patients (64%), with 4 of 21 stenoses (19%) diagnosed by catheterization identified; similarly, the right PA was imaged in 81 (62%), and 3 of 17 stenoses (18%) were identified. The distal aortic arch was visualized in 104 (80%), with successful identification of 21 of 27 (78%) of coarctations diagnosed by catheterization. Complete vascular echocardiography (visualization of PAs and aortic arch) occurred in 43% and was not obtained more frequently with sedation. Conclusions—In a large cohort of patients presenting for BDG, evaluation by echocardiography frequently failed to image the PAs and missed the majority of PA stenoses. Sedation did not appear to improve the performance of echocardiography for evaluation of the PAs. Echocardiography cannot be relied on as the sole investigation before BDG. (Circ Cardiovasc Imaging. 2011;4:498-505.) Key Words: congenital heart disease 䡲 echocardiography 䡲 single ventricle 䡲 superior cavopulmonary connection P atients with congenital heart disease with single-ventricle physiology typically undergo staged surgical palliation culminating in the Fontan procedure. Bidirectional Glenn operation (BDG) is often performed in infancy as an intermediate procedure, and results in passive flow of venous return from the upper body through the pulmonary vascular bed. Impedance to blood flow through the pulmonary circulation leads to poor postoperative outcomes after BDG.1–3 In addition, the presence of aortic arch obstruction is a risk factor for late mortality after stage I Norwood operation.4 –5 Therefore, adequate evaluation of this vascular anatomy is required before surgery. ever, with marked improvement in noninvasive imaging modalities including echocardiography and cardiac magnetic resonance, as well as the technical difficulty, expense, and ionizing radiation exposure of catheterization, there has been increasing interest in noninvasive evaluation alone.7,8 Retrospective studies have demonstrated that patients are rarely excluded from BDG on the basis of findings at catheterization, and that routine catheterization after an unremarkable echo is of limited clinical utility.7,9 A recent randomized, prospective study of cardiac magnetic resonance versus catheterization before BDG showed similar outcomes, fewer minor complications, and lower hospital charges with cardiac magnetic resonance.10 However, cardiac magnetic resonance is resource-intensive, technically challenging, and requires general anesthesia in this patient population. These findings raise the question of whether echo may be used as the sole preoperative imaging modality. We therefore sought to evaluate the accuracy and reliability of echo in this setting as compared with the gold standard Clinical Perspective on p 505 Cardiac catheterization has been performed routinely in the evaluation of patients presenting for BDG to assess for hemodynamic and anatomic issues that might complicate or exclude patients from BDG, including the assessment of the pulmonary vascular anatomy, and to intervene on such conditions with transcatheter therapy if appropriate.6 How- Received January 3, 2011; accepted June 21, 2011. From the Department of Cardiology, Children’s Hospital Boston and Department of Pediatrics, Harvard Medical School, Boston, MA. Guest Editor for this article was Craig E. Fleishman, MD. Correspondence to David W. Brown, MD, Department of Cardiology, Children’s Hospital, 300 Longwood Ave, Boston, MA 02115. E-mail [email protected] © 2011 American Heart Association, Inc. Circ Cardiovasc Imaging is available at http://circimaging.ahajournals.org 498 DOI: 10.1161/CIRCIMAGING.110.963280 Stern et al Echo Before Glenn Operation 499 Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 Figure 1. Location of pulmonary artery measurements on echocardiography. A, Right pulmonary artery behind superior vena cava and central pulmonary artery behind ascending aorta. B, Left pulmonary artery anterior to descending aorta. of catheterization, with particular attention to vascular anatomy and dimensions, and to evaluate the relationship between patient or procedural factors and echo performance. Methods Patients who underwent BDG at Children’s Hospital Boston from January 2004 to April 2008 were identified by search of the Cardiovascular Program database. Patients were included for analysis if they had both echo and catheterization performed before BDG and had images available for review in our digital imaging database. Patient demographic, clinical, and procedural variables were collected for analysis. Pre-BDG studies were defined as those echos and catheterizations performed ⱕ60 days of BDG. Echo did not have to precede catheterization to be considered the pre-BDG study. For patients with multiple echos during this period, the most complete study was selected as the pre-BDG study. The study was approved by the Department of Cardiology Scientific Review Committee and by the Children’s Hospital Boston Institutional Review Board, with waiver of informed consent. Echocardiography Digital images from pre-BDG echos were reviewed by an investigator blinded to the results of other imaging studies or intraoperative findings (K.S.). Blinding was achieved by the institution of a temporal gap of approximately 6 months between review of catheterization angiograms and echo images. Intraoperative findings were reviewed last after having collected catheterization and echo data. Images were evaluated for visualization of the structure of interest, and measurements of vascular structures were made to the nearest 0.1 mm. Branch pulmonary artery (PA) z-scores were recorded. A second investigator (D.W.B.) blinded to the results of other imaging studies repeated measurements and assessment of central PA distortion on a randomly selected 10% sample to assess interobserver variability. Earlier echos in the sample were obtained with HP SONOS 7500 and 5500 ultrasound scanners (Hewlett-Packard Co, Palo Alto, CA), and later studies were obtained with the Philips IE33 (Philips Medical Systems, Andover, MA). Images were reviewed on HeartSuite Vericis image review software (Emageon, Birmingham, AL). Complete vascular echo was defined as adequate visualization of the central PA, mediastinal branch PAs, distal aortic arch and proximal descending aorta. Visualization of the central PA and mediastinal branch PAs was typically from parasternal imaging views (Figure 1). The mediastinal right PA (RPA) was measured at the level of the right superior vena cava, the central PA in the midline typically behind the ascending aorta, and the mediastinal left PA (LPA) at the level of the descending aorta. The central PA was assessed qualitatively for degree of distortion, which was defined as abnormal tortuosity, and characterized as undistorted, minor, or major distortion. If stenosis was present in the central or branch PAs, the narrowest diameter and the adjacent unobstructed segment were measured and percent stenosis was calculated. Stenoses were categorized as no significant stenosis (⬍25%) or mild (25% to 50%), moderate (51% to 75%), or severe (⬎75%), in accordance with the previously published criteria.9 Echo assessment of the aortic arch was typically obtained from high parasternal or suprasternal notch imaging views. Measurements were made at the narrowest portion of the aortic isthmus and at the adjacent unobstructed portion of the proximal descending aorta. Doppler gradients corrected for proximal velocity across the distal aortic arch were categorized as unobstructed (⬍10 mm Hg) or obstructed (ⱖ10 mm Hg). Catheter Angiography Pre-BDG catheterization digital angiograms were reviewed by an investigator blinded to the results of the echo images (K.S.). Angiograms were assessed for visualization of the structures of interest, and vascular measurements were made to the nearest 0.1 mm with the use of electronic calipers on HeartSuite Vericis image review software version 6.20.1 (Emageon, Birmingham, AL). For comparison of branch PA hypoplasia with echo, branch PA z-scores were also calculated for angiographic measurements. A second investigator (D.W.B.), blinded to the results of other studies, performed repeat measurements and assessment of central PA distortion of catheter angiograms on a randomly selected 10% sample to assess for interobserver variability. Qualitative assessment of the degree of distortion of the central PA was reported as undistorted, minor, or major distortion (Figure 2). Measurements of PA angiograms were preferentially made in the AP projection with digital calibration. Measurements were performed at the same location as on echo. For stenoses, the narrowest diameter was measured as well as the immediately adjacent nonstenotic segment. For the distal aortic arch, the absolute diameter of the narrowest segment of the distal arch was measured preferentially through the lateral projection, with measurement of the adjacent unobstructed proximal descending aorta. For patients who underwent intervention on the PAs or the aortic arch, catheterization measurements were made either before or after intervention, depending on whether the pre-BDG echo was a precatheterization or postcatheterization study. For example, a patient undergoing balloon dilation of the RPA with a postcatheterization echo had postintervention catheterization measurements used for comparison with echo and was not excluded from analysis. 500 Circ Cardiovasc Imaging September 2011 Figure 2. Pulmonary artery distortion assessment. Catheter angiograms demonstrating examples of A, no distortion; B, mild distortion; and C, major distortion of the central pulmonary artery. Statistical Analysis Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 Patient characteristics were compared for subjects with complete versus incomplete vascular echos using the Wilcoxon rank sum test for continuous variables and Fisher exact test for categorical variables. Categorical variables measured by both echo and catheterization were cross-tabulated, and percent agreement of echo with catheterization was calculated. For continuous variables measured by both techniques, analysis was performed only on patients who had echo and catheterization performed not more than 14 days apart; a 95% confidence interval for the mean difference was calculated, and statistical significance was assessed using the paired t test. Analyses were performed overall and then separately for sedated and unsedated echos. Differences were illustrated using the Bland-Altman method. Associations between echo and catheterization measurements were also quantified using the Pearson correlation coefficient. For a random sample of 10% of study subjects, interobserver variability was assessed for both echo and catheterization measurements by calculating a 95% confidence interval for the mean difference between observers and by the paired t test. Analyses were performed using Stata version 10 (StataCorp LP, College Station, TX). Results A total of 207 patients underwent BDG at Children’s Hospital Boston from January 2004 through April 2008. From this population, 130 patients who had both pre-BDG echo and catheterization performed within 60 days of surgery were included. The majority of exclusions were for lack of digital catheterization (n⫽38) or echo (n⫽28) images. Baseline patient characteristics are shown in Table 1. Median age and weight at time of echo were 4 months and 5.7 kg, respectively; median time between pre-BDG echo and catheterization was 1 day. The time difference from echo to catheterization ranged from 55 days before to 57 days after; however, 116 studies took place within 14 days of each other, with the majority (n⫽100) on either the same or consecutive days. Most of the echos (n⫽102) were performed before the catheterization. In a sample of 10% of study patients, there were no significant differences between reviewers for either echo or catheterization measurements of the PAs, distal aortic arch, or proximal descending aorta. Agreement on central PA distortion was 100%. Echo Completeness A complete vascular echo was obtained in 56 patients (43%). No patient characteristics were associated with the likelihood of obtaining a complete vascular echo, including anatomic diagnosis and prior interventions. Timing of echo relative to catheterization was not significantly related to obtainment of a complete vascular echo (Table 2). Echo Assessment of Branch PAs The LPA was imaged by echo in 83 (64%) patients. LPA stenosis was present by catheterization in 21 patients (16%): mild in 12 (9%), moderate in 8 (6%), no severe stenoses, and 1 case of LPA atresia (1%). For stenoses, 5 of 21 cases were identified by echo, 4 of which were accurately categorized by severity. For mild LPA stenosis, 2 of 12 cases were correctly Table 1. Patient Characteristics (nⴝ130) Variable No. (%) or Median (Range) Sex Male 71 (55%) Female 59 (45%) Age, mo* 4 (1 to 61) Height, cm, n⫽84 62 (47 to 82) Weight, kg 5.7 (2.4 to 19.7) Time from echo to catheterization, d 1 (⫺57 to 55) Anatomic type of single ventricle Hypoplastic left heart syndrome 71 (55%) Complex 2-ventricle 14 (11%) Double-inlet left ventricle 11 (8%) Single right ventricle 11 (8%) Tricuspid atresia 9 (7%) Unbalanced AV canal 8 (6%) Pulmonary atresia with intact ventricular septum 6 (5%) Previous surgery Stage I/RVPA conduit 46 (35%) Stage I/modified BTS 37 (28%) Modified BTS alone 21 (16%) No prior operation 11 (8%) Main pulmonary artery banding 6 (5%) Modified BTS and TAPVC repair 4 (3%) Hybrid palliation 2 (2%) Central shunt 2 (2%) RVOT patch and modified BTS 1 (1%) AV indicates atrioventricular; RVPA, right ventricle to pulmonary artery; BTS, Blalock-Taussig shunt; TAPVC, total anomalous pulmonary venous connection; and RVOT, right ventricular outflow tract. *Age, height, and weight are from time of echo. Stern et al Table 2. Factors Associated With a Complete Vascular Echocardiogram Complete (n⫽56, 43%) Incomplete (n⫽74, 57%) Sex 0.16 Male 35 (62%) 36 (49%) Female 21 (38%) 38 (51%) Age, mo Height, cm, n⫽34, 50 Weight, kg BSA, m2 Time echo to catheterization, d 5 (1–32) 5 (2–61) 0.80 61 (54–72) 62 (47–82) 0.43 identified by echo, 2 of which were accurately categorized. None of the 10 cases of mild RPA stenosis were identified by echo (5 not visualized, 5 no stenosis). For the 7 cases of moderate RPA stenosis, only 2 were diagnosed by echo (3 not visualized, 1 no stenosis, 1 mild stenosis). All 3 cases of RPA stenosis identified by echo were confirmed by catheterization. In the 81 studies in which the RPA was adequately imaged, agreement with catheterization was 91% (n⫽74), though 6 stenoses were missed. 5.7 (2.4–12.0) 5.6 (2.4–19.7) 0.52 Echo Assessment of Central PA 0.31 (0.18–0.73) 0.51 1 (⫺57 to 51) 0 (⫺29 to 55) 0.69 The central PA was imaged by echo in 105 (81%) patients. Of the 105 visualized by echo, agreement with catheterization for assessment of degree of central PA distortion was 66% (n⫽69). By catheterization, 82 central PAs were undistorted; by echo, 59 of these were similarly categorized as undistorted, 4 were categorized as minor distortion, and 19 could not be visualized. Minor central PA distortions were diagnosed in 35 by catheterization; by echo, 8 were successfully identified, 19 were described as no distortion, 1 as major distortion, and 7 could not be visualized. Of the 13 major distortions diagnosed by catheterization, 2 were successfully identified by echo, 9 were labeled as no distortion, and 2 as mild distortion. There were 45 discrete stenoses of the central PA diagnosed by catheterization. By echo, 37 central PAs were identified as stenotic; however, only 20 of these stenoses were corroborated on catheterization, and the other 17 were mislabeled by echo as stenotic. Overall, the agreement of echo with catheterization in appropriately identifying presence or absence of central PA stenosis was 59% (n⫽77). 0.28 Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 Before catheterization 41 (73%) 61 (82%) After catheterization 15 (27%) 13 (18%) 34 (61%) 37 (50%) Complex 2V 3 (5%) 11 (15%) DILV 6 (11%) 5 (7%) Anatomic diagnosis 0.60 Single RV 5 (9%) 6 (8%) Tricuspid atresia 3 (5%) 6 (8%) Unbalanced CAVC 3 (5%) 5 (7%) PA/IVS 2 (4%) 4 (5%) 34 (61%) 49 (66%) Systemic shunt 9 (16%) 19 (26%) None 8 (14%) 3 (4%) Other* 5 (9%) 3 (4%) Prior operation Stage I 501 0.32 (0.18–0.52) Echo timing HLHS P Value Echo Before Glenn Operation 0.09 Sedation status Echo Assessment of the Aortic Arch 0.72 Sedated study 30 (54%) 43 (58%) Unsedated study 26 (46%) 31 (42%) BSA indicates body surface area; HLHS, hypoplastic left heart syndrome; 2V, 2-ventricular anatomy; DILV, double-inlet left ventricle; RV, right ventricle; CAVC, common atrioventricular canal; and PA/IVS, pulmonary atresia with intact ventricular septum. Data are depicted as No. (%) or median (range). *Includes main pulmonary artery banding (n⫽6) and bilateral pulmonary artery banding and stenting of the ductus arteriosus as part of hybrid stage I palliation for hypoplastic left heart syndrome (n⫽2). diagnosed by echo (8 not visualized, 1 no stenosis, 1 moderate stenosis). For moderate LPA stenosis, 2 of 8 cases were diagnosed by echo (2 not visualized, 4 no stenosis). The single case of LPA atresia was not identified by echo. Of the 6 cases of LPA stenosis identified by echo, 5 were confirmed by catheterization and 1 was not. In the 83 studies in which the LPA was adequately imaged, agreement with catheterization for presence or absence of stenosis was 92% (n⫽76), though 5 stenoses were missed. The RPA was imaged by echo in 81 (62%) patients. RPA stenosis was present by catheterization in 17 patients (13%): mild stenosis in 10 (8%), moderate stenosis in 7 (5%), no severe stenosis or atresia. Of 17 RPA stenoses, only 3 were The distal aortic arch was imaged adequately by echo in 104 (80%) studies. The proximal descending aorta was visualized on 102 (78%) studies. The distal aortic arch was adequately imaged by catheterization in 128 patients; 27 aortic arch obstructions were diagnosed. Of these, 21 were identified by echo and 6 were not (3 not visualized, 3 labeled as unobstructed.) Of the 103 studies in which the aortic arch was imaged by both echo and catheterization, agreement with regard to presence or absence of obstruction was 83% (85 of 103). Echo tended to overestimate arch obstruction, with 15 unobstructed arches labeled as obstructed. Vascular Measurements Table 3 and Table 4 summarize the echo and catheterization measurements of the PAs and aortic arch. Compared with catheterization, the diameters of the PAs measured by echo were generally smaller. The mean LPA measurement by echo was 5.5 mm and by catheterization 6.2 mm (P⫽0.004) (Figure 3). Similarly, the mean RPA measurement by echo was 5.7 mm and by catheterization was 6.6 mm (P⫽0.001) (Figure 4). For the assessment of branch PA hypoplasia, there were 23 RPA measurements by echo that resulted in a z-score ⬍⫺2. Only 3 of these measurements had corresponding catheterization measurements that also resulted in a z-score ⬍⫺2. For 502 Table 3. Circ Cardiovasc Imaging September 2011 Unobstructed Branch Pulmonary Artery Measurements All studies Sedated studies Unsedated studies Location of Measurement n Echo, mm, Mean⫾SD Catheterization, mm, Mean⫾SD Mean Difference, mm, Mean⫾SD 95% Confidence Interval P Value Pearson r* RPA 67 5.7⫾1.8 6.6⫾2.4 ⫺0.9⫾2.1 (⫺1.4, ⫺0.4) 0.001 0.56 LPA 68 5.5⫾1.9 6.2⫾2.4 ⫺0.7⫾1.7 (⫺1.0, ⫺0.2) 0.004 0.72 CPA 89 6.2⫾3.2 6.5⫾3.4 ⫺0.3⫾2.2 (⫺0.7, 0.2) 0.28 0.78 RPA 35 5.7⫾1.7 6.7⫾2.5 ⫺1.0⫾2.1 (⫺1.7, ⫺0.3) 0.01 0.55 LPA 41 5.5⫾1.6 6.4⫾2.3 ⫺0.9⫾1.9 (⫺1.5, ⫺0.3) 0.005 0.58 CPA 52 5.9⫾2.8 6.3⫾3.3 ⫺0.4⫾2.2 (⫺1.0, 0.2) 0.24 0.76 RPA 32 5.7⫾2.0 6.5⫾2.4 ⫺0.7⫾2.1 (⫺1.5, 0.03) 0.06 0.57 LPA 27 5.6⫾2.3 5.8⫾2.5 ⫺0.2⫾1.1 (⫺0.6, 0.3) 0.41 0.90 CPA 37 6.6⫾3.8 6.7⫾3.6 ⫺0.1⫾2.2 (⫺0.9, 0.6) 0.78 0.82 RPA indicates right pulmonary artery; LPA, left pulmonary artery; and CPA, central pulmonary artery. *P values for Pearson correlation are all statistically significant (P⬍0.001). Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 the LPA, there were 19 measurements by echo that resulted in z-scores ⬍⫺2; of these, 6 were confirmed by catheterization. Agreement for central PA measurements was better. The mean central PA measurement by echo was 6.2 mm and by catheterization was 6.5 mm (P⫽0.28). The size of the distal aortic arch was underestimated by echo, with a mean of 6.1 mm versus 6.5 mm by catheterization (P⫽0.03, Figure 5). Echo and catheterization measurements of the proximal descending aorta were similar. Sedation and Echo Timing The use of sedation was not significantly associated with increased frequency of complete echocardiograms. For the LPA, unsedated echo measurements appeared to correlate better with catheterization than sedated measurements. There was no such effect noted for the RPA or central PA (Table 3). Measurements of the distal aortic arch were more similar to catheterization in echos performed under sedation; there was no such significant difference for the proximal descending aorta (Table 4). Rates of visualization of vascular structures were not significantly different in echos performed before versus after catheterization. Rates of stenosis of the PAs, prevalence of central PA distortion, and aortic arch obstruction by catheterization were also similar between the 2 groups (Table 5). Interventions Twenty-three patients with catheterization interventions had precatheterization echos: 8 underwent balloon dilation of the Table 4. PAs and 17 underwent balloon dilation of the aortic arch. Two patients had both the aortic arch and PAs dilated. Of the 8 patients with PA dilations, only 1 had an echo on which PA stenosis was identified (3 not identified, 4 not visualized). Of the 17 patients with arch dilations, 13 had arch obstruction identified on echo (1 no obstruction, 3 not visualized). Interventions to augment the PAs at time of BDG were significantly associated with PA stenosis as well as central PA distortion. Of those who had surgical PA augmentation, 87% had PA stenosis present on catheterization, versus 29% in those without such interventions (P⬍0.001). Patients with either mild or major central PA distortions were more likely to undergo PA intervention (20 of 35 with mild distortion, 9 of 13 with major distortion; P⫽0.001). Twelve patients had central PA distortion without central or branch PA stenosis. Of these 12, 1 had an intervention on the PAs at the time of BDG in the form of PA plasty without patch material (rate of intervention on the PAs, 8%). This rate of intervention on the PAs was significantly lower than for patients with both central PA distortion and at least 1 PA stenosis (P⬍0.001). Of the 29 patients with branch PA stenosis not identified on echo, all but 3 had surgical interventions to augment the PAs at time of BDG. One of these 3 had balloon dilation of the LPA at time of catheterization. Of the 6 patients with aortic arch obstruction not identified on echo, 3 had no arch intervention at time of BDG, all of whom underwent balloon angioplasty of the coarctation at time of catheterization. Distal Aortic Arch and Proximal Descending Aorta Measurements All studies Sedated studies Unsedated studies Location of Measurement n Echo, mm, Mean⫾SD Catheterization, mm, Mean⫾SD Mean Difference, mm, Mean⫾SD 95% Confidence Interval P Value Pearson r* Distal arch 91 6.1⫾1.8 6.5⫾1.9 ⫺0.4⫾1.5 (⫺0.7, ⫺0.03) 0.03 0.65 Proximal AoDt 89 7.6⫾1.4 8.0⫾2.0 ⫺0.4⫾1.9 (⫺0.8, 0.02) 0.06 0.41 Distal arch 52 6.2⫾1.7 6.5⫾1.8 ⫺0.2⫾1.6 (⫺0.7, 0.2) 0.29 0.56 Proximal AoDt 50 7.6⫾1.4 8.0⫾2.1 ⫺0.5⫾2.1 (⫺1.0, 0.1) 0.13 0.38 Distal arch 39 6.0⫾2.1 6.5⫾2.0 ⫺0.5⫾1.5 (⫺1.0, ⫺0.03) 0.04 0.75 Proximal AoDt 39 7.7⫾1.5 8.0⫾1.8 ⫺0.3⫾1.7 (⫺0.8, 0.3) 0.31 0.45 AoDt indicates descending aorta. *P values for Pearson correlation are all statistically significant (Pⱕ0.007). Stern et al Figure 3. Bland-Altman plot of right pulmonary artery measurements. Solid line represents the mean difference; dashed lines represent the limits of agreement. Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 There were 17 patients in the cohort with no prior surgical interventions on the branch PAs (no prior operation or main PA banding only). Of these 17, there were 2 cases of RPA stenosis and 2 cases of LPA stenosis diagnosed by catheterization on 4 separate patients. Three of these patients had a main PA band as their prior operation, but 1 patient had no previous operations. Two of these patients underwent patch PA plasty at time of BDG (including the patient with no prior operation), and a third had the Glenn anastomosis created in such a way to relieve PA stenosis. Discussion In this large, retrospective study of BDG candidates with a broad range of cardiac diagnoses and prior surgical interventions, echo showed generally suboptimal performance for the evaluation of the relevant vascular anatomy. Echo was frequently unable to adequately image the mediastinal branch PAs and missed the majority of branch PA stenoses. When visualized, there were statistically significant differences between measurements for dimensions of the branch PAs, with general underestimation of the diameter of both branch PAs by echo. Although the differences in individual cases were occasionally large as demonstrated by the BlandAltman figure, the average difference across the cohort was generally ⬍1 mm. In general, the use of sedation did not appear to improve the performance of echo for these factors. The ability of echo to image the central PA was better than for the branch PAs. Moreover, echo measurements of the central PA were more similar to those obtained by catheterization. Echo Before Glenn Operation 503 Figure 5. Bland-Altman plot of distal aortic arch measurements. Solid line represents the mean difference; dashed lines represent the limits of agreement. Sedated and unsedated measurements are differentiated by icons. However, agreement in identifying presence or absence of stenosis was only 59%. Moreover, agreement in the categorization of the degree of distortion of the central PA was generally poor, and echo missed the majority of cases of major distortion. Distal aortic arch measurements by echo tended to be underestimates as compared with catheterization, but the use of sedation improved echo performance. Echo successfully diagnosed 78% of aortic arch obstructions diagnosed by catheterization. Previous studies investigating the accuracy of echo imaging of the PAs in congenital heart disease have had conflicting results and have been performed in a variety of different lesions. Huhta et al11 compared echo with catheterization in 65 patients with pulmonary atresia with a ventricular septal defect and found that the LPA was adequately imaged in only Table 5. Echocardiogram Performance and Catheterization Findings Echo All (n⫽130) Before Catheterization (n⫽102) After Catheterization (n⫽28) P Value Visualization by echo, n (%) LPA 83 (64) 65 (64) 18 (64) 1 RPA 81 (62) 63 (62) 18 (64) 1 CPA 105 (81) 83 (81) 22 (79) 0.79 DAA 104 (80) 82 (80) 22 (79) 0.8 Proximal descending aorta 102 (78) 79 (77) 23 (82) 0.8 Findings at catheterization, n (%) Figure 4. Bland-Altman plot of left pulmonary artery measurements. Solid line represents the mean difference; dashed lines represent the limits of agreement. LPA stenosis 21 (16) 17 (17) 4 (14) RPA stenosis 17 (13) 15 (15) 2 (7) 1 0.36 CPA stenosis 45 (35) 37 (36) 8 (29) 0.51 CPA distortion 48 (37) 40 (39) 8 (29) 0.38 Aortic arch obstruction 27 (21) 24 (24) 3 (11) 0.19 LPA indicates left pulmonary artery; RPA, right pulmonary artery; CPA, central pulmonary artery; and DAA, distal aortic arch. 504 Circ Cardiovasc Imaging September 2011 Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 16 of 65 (25%), but the RPA was visualized in 55 of 65 (85%), and echo measurements correlated well with angiography. Gutgesell et al12 prospectively studied 20 cyanotic infants with echo before catheterization and compared the ability of echo to visualize and measure the central PA, branch PAs and aortic arch. Vessel size was slightly underestimated though these structures were visualized readily. Fogel et al13 compared echo and MRI with catheterization in 36 patients with single ventricle across different stages of Fontan palliation, including 16 pre-BDG. They found more significant variation in PA measurements with echo than with MRI.13 Greenberg et al14 compared echo with MRI in 20 patients after repair for tetralogy of Fallot and found that echo performed poorly, with the right and left branch PAs not visualized in 8 and 10 children, respectively. Stenoses were also missed in 13 branch PAs. Even given this history of echo’s suboptimal performance, the rate of incomplete echocardiograms in the current study was higher than in previous studies. In prior reports from this institution, higher rates of visualization of vascular structures are described, ranging from 73% to 93%.9,10 However, the previous studies involved smaller, less contemporaneous cohorts. In addition, echocardiograms for prior studies were likely less focused on details of the vascular anatomy, and were interpreted by multiple different clinical staff. Secondary review of primary image data with regard to all vascular structures was not necessarily performed. Furthermore, the process of making independent blinded measurements of vascular dimensions in the current report likely lowered the threshold for nonvisualization of a structure, resulting in a lower rate of complete echos. Analyzing echos in which the structure of interest was visualized, agreement between echo and catheterization on PA stenosis was ⬎90% for both right and left PAs. However, this number is misleading because 5 LPA and 6 RPA stenoses were still missed. A false sense of reassurance can be created if a complete echocardiogram is obtained and no PA stenoses are identified. The current study revealed equally poor performance for both RPA and LPA visualization. Prior literature describes better performance for echo imaging of the RPA as compared with the LPA.11,15 One possible explanation for this discrepancy is the higher prevalence of systemic to PA shunts in the current cohort. A Blalock-Taussig shunt or Sano conduit was present in 84% of patients. Because these shunts are often inserted onto the RPA, this could hinder visualization of this structure. Reliable assessment of PA distortion is difficult because of its qualitative nature. PA distortion has been defined in the literature as “distorted or markedly hypoplastic peripheral or central pulmonary arteries”16 as well as the nonuniform growth and development of the PAs.17 PA distortion has been shown to increase the risk of death or takedown of Fontan repair and is considered an independent risk factor for mortality.16,18 We found an increased rate of surgical interventions on the PAs in patients with central PA distortion; however, when patients with PA stenoses were eliminated from this group, the rate of intervention fell to 1 of 12, or 8%. It is possible that central PA distortion may represent a surrogate for PA stenosis because patients with distortion and no stenosis had a significantly decreased rate of surgical intervention on the branch PAs. The small number of patients without previous surgical interventions on the branch PAs limits our conclusions from these data, though they do suggest that suspicion for PA stenoses should be high in all BDG candidates. In addition, surgeons at our institution do not routinely dissect the branch PAs out completely at time of BDG or use intraoperative angiography. Therefore, thorough knowledge of branch PA anatomy adds significantly to the surgical planning process. A complete anatomic assessment of the branch PAs with a modality sufficiently sensitive to detect stenoses should be performed in all patients undergoing BDG, regardless of underlying anatomy or prior interventions. Alternatively, intraoperative angiography may be used at time of BDG to assess the PAs. The performance of echo for the evaluation of the aortic arch in this setting, particularly in those with prior stage I palliation with aortic reconstruction, has been well described.5,19 Our study confirmed echo as a reasonably good screening tool for arch obstruction, diagnosing 21 of 27 cases. However, it also predicted arch obstruction in many patients that was not present at catheterization. Some of this probably is due to the typical increase in flow velocity across the distal reconstructed aortic arch, which can result in the false impression of arch obstruction. Echo has a major role in the pre-BDG evaluation of the atrial septum, the degree and mechanism of valvular regurgitation, and the evaluation of ventricular function. All of these factors are crucial to the short- and long-term outcomes after BDG. However, this study demonstrates the limitations of echo in its ability to visualize mediastinal vascular structures, particularly the branch PAs. There are no readily identifiable methods available to improve the performance of echo in this regard because the limitations probably are due mostly to acoustic interference by lung tissue. It is clear that echo alone cannot be relied on as the sole investigation before BDG. We suggest using echo as part of a multimodality imaging evaluation, with techniques such as cardiac magnetic resonance, catheterization or intraoperative angiography used to further delineate the relevant vascular anatomy. Limitations This study is prone to all the limitations inherent in retrospective investigations. Because the diagnostic studies were not performed with the intention of being the sole preoperative imaging test, it is difficult to ascertain whether a prospective study with this intent would yield substantially different findings. However, at our institution, both echo and catheterization are approached with the intent of evaluating all relevant structures in detail, regardless of other planned studies. Additionally, surgical intervention at time of BDG was used as a surrogate for clinical severity, though it is not certain that many minor PA stenoses warrant intervention. Conclusions In a large cohort of patients presenting for BDG, echo evaluation frequently failed to image the mediastinal branch Stern et al PAs, missed the majority of branch PA stenoses, and underestimated branch PA size. Sedation did not appear to improve the performance of echo for the branch PAs. The aortic arch was visualized more readily and the majority of aortic arch obstructions were identified by echo; however, arch obstruction tended to be overestimated on echo. Echo cannot be relied on as the sole investigation before BDG. Other diagnostic imaging tools such as cardiac magnetic resonance or catheterization angiography appear to still be necessary for complete visualization of the relevant vascular anatomy. Disclosures None. References Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 1. Fontan F, Fernandez G, Costa F, Naftel DC, Tritto F, Blackstone EH, Kirklin JW. The size of the pulmonary arteries and the results of the Fontan operation. J Thorac Cardiovasc Surg. 1989;98:711–719. 2. Knott-Craig CJ, Danielson GK, Schaff HV, Puga FJ, Weaver AL, Driscoll DJ. The modified Fontan operation: an analysis of risk factors for early postoperative death or takedown in 702 consecutive patients from one institution. J Thorac Cardiovasc Surg. 1995;109:1237–1243. 3. Choussat A, Fontan F, Besse P, Vallot F, Chauve A, Bricaud H. Selection criteria for Fontan procedure. In: Anderson RH, Shinebourne EA, eds. Pediatric Cardiology. Edinburgh: Churchill Livingstone; 1978:559 –566. 4. Jonas RA. Intermediate procedures after first-stage Norwood operation facilitate subsequent repair. Ann Thorac Surg. 1991;52:696 –700. 5. Fraisse A, Colan SD, Jonas RA, Gauvreau K, Geva T. Accuracy of echocardiography for detection of aortic arch obstruction after stage I Norwood procedure. Am Heart J. 1998;135:230 –236. 6. Nakanishi T. Cardiac catheterization is necessary before bidirectional Glenn and Fontan procedures in single ventricle physiology. Pediatr Cardiol. 2005;26:159 –161. 7. McMahon CJ, Eidem BW, Bezold LI, Vargo T, Neish SR, Bricker JT, Kovalchin J, El-Said H. Is cardiac catheterization a prerequisite in all patients undergoing bidirectional cavopulmonary anastamosis? J Am Soc Echocardiogr. 2003;16:1068 –1072. 8. Fogel MA. Is routine cardiac catheterization necessary in the management of patients with single ventricles across staged Fontan reconstruction? No! Pediatr Cardiol. 2005;26:154 –158. Echo Before Glenn Operation 505 9. Brown DW, Gauvreau K, Moran AM, Jenkins KJ, Perry SB, del Nido PJ, Colan SD. Clinical outcomes and utility of cardiac catheterization prior to superior cavopulmonary anastomosis. J Thorac Cardiovasc Surg. 2003; 126:272–281. 10. Brown DW, Gauvreau K, Powell AJ, Lang P, Colan SD, Del Nido PJ, Odegard KC, Geva T. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis in infants with functional single ventricle: a prospective randomized trial. Circulation. 2007;116:2718 –2725. 11. Huhta JC, Piehler JM, Tajik AJ, Hagler DJ, Mair DD, Julsrud PR, Seward JB. Two dimensional echocardiographic detection and measurement of the right pulmonary artery in pulmonary atresia-ventricular septal defect: angiographic and surgical correlation. Am J Cardiol. 1982;49:1235–1240. 12. Gutgesell HP, Huhta JC, Cohen MH, Latson LA. Two-dimensional echocardiographic assessment of pulmonary artery and aortic arch anatomy in cyanotic infants. J Am Coll Cardiol. 1984;4:1242–1246. 13. Fogel MA, Donofrio MT, Ramaciotti C, Hubbard AM, Weinberg PM. Magnetic resonance and echocardiographic imaging of pulmonary artery size throughout stages of Fontan reconstruction. Circulation. 1994;90: 2927–2936. 14. Greenberg SB, Crisci KL, Koenig P, Robinson B, Anisman P, Russo P. Magnetic resonance imaging compared with echocardiography in the evaluation of pulmonary artery abnormalities in children with tetralogy of Fallot following palliative and corrective surgery. Pediatr Radiol. 1997; 27:932–935. 15. Vick GW, Rokey R, Huhta JC, Mulvagh SL, Johnston DL. Nuclear magnetic resonance imaging of the pulmonary arteries, subpulmonary region, and aorticopulmonary shunts: a comparative study with twodimensional echocardiography and angiography. Am Heart J. 1990;119: 1103–1110. 16. Mayer JE, Bridges ND, Lock JE, Hanley FL, Jonas RA, Castaneda AR. Factors associated with marked reduction in mortality for Fontan operations in patients with single ventricle. J Thorac Cardiovasc Surg. 1992; 103:444 – 452. 17. Jonas RA. Intermediate procedures after first-stage Norwood operation facilitate subsequent repair. Ann Thorac Surg. 1991;52:696 –700. 18. Bridges ND, Jonas RA, Mayer JE, Flanagan MF, Keane JF, Castaneda AR. Bidirectional cavopulmonary anastamosis as interim palliation for high-risk Fontan candidates. Circulation. 1990;82(suppl IV):IV170 –IV-176. 19. Sekar P, Border WL, Kimball TR, Hirsch R, Manning PB, Khoury PR, Beekman RH III. Aortic arch recoarctation after the Norwood stage I palliation: the comparative accuracy of blood pressure cuff and echocardiographic Doppler gradients in detecting significant obstruction. Congenit Heart Dis. 2009;4:440 – 447. CLINICAL PERSPECTIVE There has been increasing interest in noninvasive evaluation alone in patients with single-ventricle circulation before undergoing bidirectional Glenn operation. We studied the ability of echocardiography to visualize and assess the relevant vascular anatomy in this patient population. Echocardiography was found to perform poorly compared with catheter angiography. The branch pulmonary arteries were successfully imaged by echocardiography in under two-thirds of patients, and the majority of pulmonary artery stenoses found at catheterization were not visualized by echocardiography. The aortic arch was imaged more readily by echocardiography, with the majority of arch obstructions identified. Sedation did not appear to improve the performance of echocardiography for assessment of the pulmonary arteries. Given the clinical importance of identification and treatment of obstructions to pulmonary blood flow in the single ventricle circulation, we conclude that echocardiography alone before bidirectional Glenn operation is insufficient to image the relevant vascular anatomy. Echocardiographic Evaluation Before Bidirectional Glenn Operation in Functional Single-Ventricle Heart Disease: Comparison to Catheter Angiography Kenan W.D. Stern, Doff B. McElhinney, Kimberlee Gauvreau, Tal Geva and David W. Brown Downloaded from http://circimaging.ahajournals.org/ by guest on May 11, 2017 Circ Cardiovasc Imaging. 2011;4:498-505; originally published online July 5, 2011; doi: 10.1161/CIRCIMAGING.110.963280 Circulation: Cardiovascular Imaging is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2011 American Heart Association, Inc. All rights reserved. Print ISSN: 1941-9651. Online ISSN: 1942-0080 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circimaging.ahajournals.org/content/4/5/498 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation: Cardiovascular Imaging can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation: Cardiovascular Imaging is online at: http://circimaging.ahajournals.org//subscriptions/