* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Structural, electronic and optical properties of TiO2 nanoparticles
Diffraction topography wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Chemical imaging wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Anti-reflective coating wikipedia , lookup
Dispersion staining wikipedia , lookup
X-ray fluorescence wikipedia , lookup
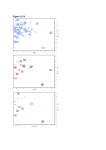
Structural, electronic and optical properties of TiO2 nanoparticles Matti Alatalo, Sami Auvinen, Heikki Haario Lappeenranta University of Technology Juho Jalava, Ralf Lamminmäki Sachtleben Pigments Outline − − − − − Motivation, earlier studies Methods: Brief description Ab initio results Simpler approaches Outlook Industrial use of TiO2 nanoparticles − TiO2 pigments are widely used in the industry: whiteness, opacity − Nano-TiO2: Plastics, coatings, cosmetics − Particle size and shape distribution important for applications − These distributions can be solved by measuring the turbidity spectrum of a dilute solution: A nontrivial inverse problem Measurement of turbidity spectrum of rutile or anatase pigments Turbidity spectra of sample (normalized to 10 mg/l): XRDI-S 483.44 21.3.05/06.30 0.25 measured calculated calculated and norm pigment + water + dispersing agent (MIPA) Light to the sample absorbance I I 0e 0.2 L 0.15 0.1 0.05 200 p1011054 TUOTEKEH.LAB. weight 0.1204 g conc. 11.33 mg/l looseness 0.2 w% 300 400 500 600 700 wavelength, nm 800 900 1000 1100 Calculation of the turbidity − When the refractive index of a material is known at different wavelengths, the turbidity can be calculated rigorously, e.g., for spheroid a m N (q, a )Cext q, − − − − − − − , np nm N is the number of particles, a is the width of spheroid q is the length/width Cext is the extinction coefficient n is the refractive index p refers to the particle and m refers to the medium Cext-matrix for spheroids as function of wavelength and crystal size diameter calculated by the T-matrix method 1.5 1.5 1 Cext Cext 1 0.5 0.5 0 600 0 600 400 400 1000 200 800 200 vol. eq. crystal size diameter, nm 1000 600 0 Length/width 1.1 400 wavelength, nm 800 vol. eq. crystal size diameter, nm 600 0 400 Length/width 2.1 wavelength, nm Limitations of the T-matrix modeling Fitting is moderate but the error in numerical results is much larger than expected. Turbidity spectra of sample (normalized to 10 mg/l): XRD: 8 nm 2 measured calculated calculated and norm absorbance 1.5 uvtsmfige8 mitattu weight conc. looseness spektrin kunto wl(max) abs(max) abs(450 nm) U/V*100 1 0.5 0 200 400 600 800 wavelength, nm 0.1000 g 10.00 mg/l -117.3 w% 7 16 0 0 (koko UV VIS IR) 278 nm 1.991 0.062 3191 1000 1200 Limitations of the T-matrix modeling − The results are not good at particle sizes below 200 nm and wavelengths below 360 nm − Quantum size effect? Methods − Structures, spectra: Density functional calculations as implemented in the GPAW code − Projector augmented wave method in real space grids − Structures, spectra: Density functional tight binding as implemented in the Hotbit code − First attempts (testing of the parametrization) − T-matrix modeling − Particle size distributions Details of the GPAW calculation − Clusters of the size 18-38 TiO2 units were carved from anatase/rutile bulk (Smaller ones composed of TiO2 molecules) − For small particles, anatase is known to be the ground state structure − The structures were allowed to relax − Several different structures per particle size were tested − Absorption spectra were calculated using time propagation TDDFT − Grid parameter h=0.17 for structural relaxations, h=0.3 for the calculation of the absorption spectra Results: Absorption spectra Atomic vs. electronic structure (TiO2)28 •Red: O •Blue: Ti Effect of structure on the adsorption spectra •A: •B: Effect of structure on the adsorption spectra •A: •B: Contributions of different directions •Note: Bulk anatase is birefringent Observations − Structure plays an important role on the absorption spectra − Longest dimension dominates − Compact structures energetically favorable Density functional tight binding, first results •Green: •GPAW •Blue: •DFTB