* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Topography of the pelvic autonomic nervous system and its potential
Survey
Document related concepts
Transcript
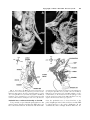
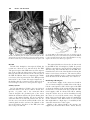
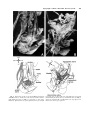
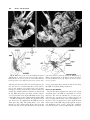
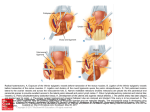
Clinical Anatomy 16:119 –130 (2003) ORIGINAL COMMUNICATION Topography of the Pelvic Autonomic Nervous System and Its Potential Impact on Surgical Intervention in the Pelvis B. BAADER* AND M. HERRMANN Department of Anatomy and Cellular Neurobiology, University of Ulm, Ulm, Germany Bladder, bowel, and sexual dysfunction caused by iatrogenic lesions of the inferior hypogastric plexus (IHP) are well known and commonly tolerated in pelvic surgery. Because the pelvic autonomic nerves are difficult to define and dissect in surgery, and their importance often ignored, we conducted a gross anatomic study of 90 adult and four fetal hemipelves. Using various non-surgical approaches, the anatomic relations and pathways of the IHP were dissected. The IHP extended from the sacrum to the genital organs at the level of the lower sacral vertebrae. It originated from three different sources: the hypogastric nerve, the sacral splanchnic nerves from the sacral sympathetic trunk (mostly the S2 ganglion), and the pelvic splanchnic nerves, which branched primarily from the third and fourth sacral ventral rami. These fibers converge to form a uniform nerve plate medial to the vascular layer and deep to the peritoneum. The posterior portion of the IHP supplied the rectum and the anterior portion of the urogenital organs; nerve fibers traveled directly from the IHP to the anterolateral wall of the rectum and to the inferolateral and posterolateral aspects of the urogenital organs. The autonomic supply from the IHP was supplemented by nerves accompanying the ureter and the arteries. An understanding of the location of the autonomic pelvic network, including important landmarks, should help prevent iatrogenic injury through the adoption of surgical techniques that reduce or prevent postoperative autonomic dysfunction. Clin. Anat. 16: 119 –130, 2003. © 2003 Wiley-Liss, Inc. Key words: pelvic plexus; hysterectomy; rectal surgery; prostatectomy; incontinence INTRODUCTION Nerve-sparing techniques are common in surgery; for example, during an axillary lymph node dissection, the thoracodorsal and long thoracic nerves are meticulously protected, except when they are directly involved by the extension of the cancer. Similarly, surgeons take great care to avoid injury to the recurrent laryngeal nerves during thyroid surgery. Although nerve protection is an indisputable objective in surgery, iatrogenic injury of the inferior hypogastric plexus (IHP) is usually accepted as the norm. After rectal resection for carcinoma, the incidence of urinary dysfunction is between 7% and 70% and sexual dysfunction between 40% and 100% (Hojo et al., 1991). Ninety percent of patients undergoing radical prostatectomy were found to be impotent (Walsh and Mostwin, 1984). These statistics clearly demonstrate the necessity for autonomic nerve preservation in pelvic surgery. © 2003 Wiley-Liss, Inc. The preservation of the pelvic autonomic nervous system is hampered by the fact that its nerves are not distinctly seen in the surgical field because of their tiny structure and the depth and narrowness of the pelvis. In addition, confusing anatomical and surgical nomenclature in this region encourages neglect of nerve-sparing techniques. For instance, although originally named a peritoneal “plica,” current nomenclature uses the term “ligamentum,” which misleadingly suggests the presence of only connective tissue ne*Correspondence to: Dr. Brigitte Baader, Department of Gynecology and Obstetrics, Frankenwaldklinik Kronach, Friesenerstrasse 41, D-96317 Kronach, Germany. E-mail: [email protected] Received 9 October 2001; Revised 16 August 2002 Published online in Wiley InterScience (www.interscience.wiley. com). DOI 10.1002/ca.10105 120 Baader and Herrmann glecting nervous tissue: the ligamentum latum uteri, the broad ligament of the uterus, contains an abundance of autonomic nerves. Pelvic nerve-sparing techniques applied in adults are based in large part on microscopic investigations of fetuses, wherein relatively thick nerves are surrounded by little connective tissue (Fritsch, 1989). A gross anatomic investigation of the IHP in adults should demonstrate to better advantage the macroscopic topography of the autonomic pelvic network and provide the necessary background for successful dissection and protection of these nerves in adult patients. Unfortunately, detailed descriptions of the pelvic autonomic nerves reported earlier (Frankenhäuser, 1867) seem to have fallen into disuse. The present topographic study provides further details concerning the relationships between pelvic autonomic nerves and surgically important structures. MATERIALS AND METHODS To investigate the anatomic location of the IHP, 67 cadavers, including 65 adults and two fetuses, were studied. A total of 94 hemipelves were dissected in the 67 cadavers: 45 male (20 right, 25 left) and 49 female (25 right, 24 left). The adult cadavers ranged in age from 38 to 98 years; the two fetuses, a male and female, were aged 24 and 23 weeks, respectively. The cadavers were preserved with a formalin-based fluid by infusion into the femoral artery. The dissections were documented by photography and hand drawings. A non-surgical laterocaudodorsal approach provided for optimal dissection of the IHP in its native visceral topography. After dissecting abdominal retroperitoneal nerves and vascular structures to the pelvic brim, the hip bones were carefully removed, piece by piece, while preserving the muscles of the pelvic floor and the supportive connective tissue associated with the subperitoneal pelvic organs. After careful dissection of the nerves and vessels of the ischio-anal fossa, the pelvic diaphragm was exposed and dissected off the pelvic viscera without damaging nervous structures. To expose the sacral ventral rami to define the origins of the pelvic splanchnic nerves, the dorsal wall and part of the lateral wall of the sacral canal were removed. The ventral rami of the spinal nerves were dissected and pelvic splanchnic branches traced to the IHP. Then, the terminal end of the dural sac and its associated spinal nerves were lifted away from the sacrum, and the anterior bony plate of the sacrum and coccyx were removed. This resulted in an exposed visceral bloc containing intact nervous connections to the spinal cord, and allowed for a detailed dissection of the autonomic innervation of the viscera. Our analysis of the autonomic innervation of the pelvic viscera of the 94 hemipelves focused on the following parameters: the origins of the contributing nerves, the location of the autonomic network, including its relationship to arteries and the pudendal nerve, and its branches to the pelvic organs. The results were either combined or divided into gender-specific groups. RESULTS Sources of the Inferior Hypogastric Plexus Contributions to an IHP arose from three different sources: from the superior hypogastric plexus via the hypogastric nerve, from the sympathetic trunk via sacral splanchnic nerves, and from ventral rami of sacral spinal nerves via pelvic splanchnic nerves. In all 67 cadavers investigated, we observed sympathetic innervation from the superior hypogastric plexus projecting to the two IHPs via the bilateral fiber bundles of the hypogastric nerves; there were no differences between male and female cadavers (Figs. 1B and 3). In addition, we were able to observe that the IHP received sympathetic input from the sacral splanchnic nerves. The dissection in Figure 1A shows a sacral splanchnic nerve originating from S2 sympathetic ganglion and contributing to the IHP. In 19 hemipelves, sacral splanchnic nerves could be identified arising from the sacral sympathetic ganglia: in 58% of these (11/19) the sacral splanchnic nerves originated primarily from the S2 sympathetic ganglion; in 15% (3/19) from the S4 ganglion; in 11% (2/19) from the S1 ganglion; in 11% (2/19) from the S3 ganglion; and in 5% of the hemipelves (1/19) from the sacral splanchnic nerves originated primarily from the S5 sympathetic ganglion. In all female cadavers, sacral splanchnic nerves derived from either S1 (14%, 1/7) or S2 (86%, 6/7) sympathetic ganglia, whereas in males all sacral sympathetic ganglia were involved. In contrast to the contributions of the sympathetic ganglia to the IHP, the parasympathetic contributions derived from sacral ventral rami of more inferior levels. In 85 hemipelves, pelvic splanchnic nerves could be identified: in 52 % of these hemipelves (44/85), the pelvic splanchnic nerves originated primarily from the ventral ramus of S3 (Fig. 1A,B); in 32% (27/85) from ventral ramus S4; in 12% (11/85) from S2; and in 4% of hemipelves (3/85) the pelvic splanchnic nerves arose primarily from ventral ramus S5. Whereas male cadavers were in congruence with these observations, the female IHP differed slightly, receiving its pelvic splanchnic nerves mainly from either ventral ramus S3 (60%, 21/35) or S4 (37%, 13/35). Topography of Pelvic Autonomic Nervous System 121 Fig. 1. Nerve fibers to the IHP in the female. A: Lateral view of pelvic viscera of a female fetus at gestational age 24 weeks, left side. B: Female adult, right side. In (A) the sympathetic trunk (1) is giving off a sacral splanchnic nerve (2) from the S2 ganglion to the IHP (4). In addition, a pelvic splanchnic nerve (3) is shown arising from ventral ramus S3. The hemostat is holding the sciatic nerve; (B) shows the sacral plexus reflected posteriorly (4) giving rise to the pudendal nerve (5) and pelvic splanchnic nerves (3). Both connect to the IHP (2). Furthermore, the most inferior parts of the IHP contribute to a neurovascular bundle (1). The hemostat shown in the right upper corner holds the superior hypogastric plexus (SHP). Orientation icons: sup, superior; inf, inferior; ant, anterior; post, posterior. Pudendal Nerve and Its Relationship to the IHP tions, the pudendal nerve is located inferior to the pelvic diaphragm in the ischio-anal fossa and the IHP is found superior to the pelvic diaphragm. In our examinations, this seemingly distinct difference be- It is generally accepted that the pudendal nerve has purely somatic function and that the IHP only regulates autonomic function. Consistent with their func- 122 Baader and Herrmann Fig. 2. Comparisons of origins of sacral parasympathetic contributions to the IHP and sacral contributions to the pudendal nerve in male (A) and female (B) pelvic cavities. Values were given as percent incidences for each sacral ventral ramus that provides a contribution to pelvic splanchnic and pudendal nerves in the male (A) and female (B) pelves (male: nparasympathetic,right ⫽ 31, nparasympathetic,left ⫽ 19; npudendal nerve,right ⫽ 8, npudendal nerve,left ⫽ 7; female: nparasympathetic,right ⫽ 22, nparasympathetic,left ⫽ 13; npudendal nerve,right ⫽ 15, npudendal nerve,left ⫽ 7). tween the topography and function of the pudendal nerve and IHP was put into question. In 37 hemipelves, sacral contributions to the pudendal nerve could be identified: in 40% of these hemipelves (15/37), the pudendal nerve derived mostly from ventral ramus S3; in 35% (13/37) from ventral ramus S4; and in 19% (7/37) from ventral ramus S2. Ventral rami L5 (3%, 1/37) and S1 (3%, 1/37) were less involved in providing the major contribution to the pudendal nerve. A comparison of the parasympathetic and the pudendal nerve origins in both males and females showed no differences. In both genders the pudendal and pelvic splanchnic nerves received fibers from S2–S4 in similar percentages (Fig. 2); for each gender somatic and autonomic nerves derived from the same sacral ventral ramus to similar degrees. In 49% of hemipelves (18/37), the pudendal nerve and pelvic splanchnic nerves’ major contribution to the IHP derived from the same ventral ramus. The typical anatomy of these nerves observed in laterocaudodorsal dissections are shown in Figures 1B and 5A. Location of the IHP Within the True Pelvis In addition to elucidating where contributing nerves to the IHP originate, it is important to investigate the exact position of the IHP. The location of the plexus was investigated in three different planes: coronal, horizontal, and sagittal. Irrespective of gender, in the horizontal plane the IHP was localized at about the level of sacral vertebrae S4 and S5. In the coronal plane, the IHP of males was located within and deep to the peritoneal fold between urinary bladder and rectum as well as pararectally. In surgery, it is important to know the percentage of cases in which the nerve fibers of the IHP can be found at a distinct position; therefore, the values given below are related to the number of instances where the IHP occurred in distinct locations, taking into account that the fibers of the IHP of one single hemipelvis can be located in two or more positions. Thus, a total of 55 locations were identified in 45 male hemipelves. In 73% of these cases (40/55), the IHP was found in the fold between urinary bladder and rectum (Fig. 3); in 27% of cases (15/55), the IHP was localized pararectally. In females, nerve fibers of the IHP were identified in four locations; a total of 60 locations were observed in 49 female hemipelves. In 57% of these cases (34/ 60), the IHP was observed in the uterosacral ligament; in 30% of cases (18/60), it was found parametrially; in only 2% (1/60) and 11% of cases (7/60) was the IHP found pararectally and in the fold between the urinary bladder and the uterus, respectively. Within the sagittal plane it is important to determine the order in which neurovascular structures are oriented between the levator ani and pelvic organs. In all 42 cases in which the orientations in the sagittal plane could be defined, the IHP was positioned immediately lateral to the pelvic organs. With respect to the position of arteries, veins, and nerves, three groups could be distinguished: in one group the veins were most lateral, followed by the arteries and the nerves (VAN, vein-artery-nerve group); the second group was composed of arteries lying lateral to the veins, which again were lateral to the nerves (AVN, artery-veinnerve group, Fig. 4); and in the last group, arteries and veins were intermingled and an exact layering could not be observed, but again the nerves were positioned most medially, giving a VN (vessel-nerve) group. The VAN order was found in 62% of IHPs (26/42) in which a clear arrangement could be determined; the VN arrangement was present in 31% of cases (13/42); and only a few hemipelves (7%, 3/42) showed an AVNordered neurovascular structure. The AVN order was only found in male cadavers (Fig. 4). Structure of the Inferior Hypogastric Plexus and Its Pattern of Innervation Compared to the variable topographic relationships of arteries and veins to the IHP, the plexus itself consistently appeared as a homogeneous nerve plate with no obvious layers (Fig. 3). Irrespective of gender, autonomic fibers emerged from the anterior part of the IHP and innervated the urogenital tract and the inferior part of the rectum (Figs. 5A and 6A). The superior part of the rectum received fibers from the posterior part of the IHP. Topography of Pelvic Autonomic Nervous System 123 Fig. 3. Position of the IHP within the pelvic cavity. Lateral view of an adult male pelvis, right side. The IHP (3) is located between the rectum (6) and the bladder (7) along a line between the bladder and coccyx (8). 1, 2, and 4 represent contributions to the IHP: pelvic splanchnic nerves (1, 2); right hypogastric nerve (4). The levator ani is labeled 5. Orientation icons: sup, superior; inf, inferior; ant, anterior; post, posterior. As shown in Figure 6A, the anterior part of the IHP was positioned in 62% of male hemipelves (28/45) in the region where the ureter, seminal gland, prostate gland, and neck of the urinary bladder converged. In about half of the female hemipelves (24/49), the bulk of the plexus was restricted to a region between the ureter, uterine artery, cervix, and urinary bladder. From the anterior part of the IHP, several groups of fibers coursed to the pelvic organs. According to their location, they were designated superior, central, and inferior branches. In females, superior branches, which mostly commingled with the ureter, were responsible for the innervation of the urinary bladder. Inferior fibers of the anterior part of the IHP innervated the inferior part of the rectum and formed the neurovascular bundle to the erectile tissue. The central branches of the anterior part of the plexus, in general, followed the course of the uterine artery to the uterine cervix (Fig. 5A). The topographical division of the female IHP was found to be similar in males; the only difference between genders was that the central portion of the anterior part of the plexus innervated the prostate and seminal gland in males. The vertical organization of the IHP followed the tilt of the pelvic viscera: the urinary bladder was located superior and the rectum inferior to the sexual organs. Thus, the upper part of the IHP innervated more ventral pelvic organs and the lower portions supplied dorsal organs. The pelvic organs, however, were also innervated by fibers that penetrated the IHP as they traveled on the ureter and arteries. Vessels As described above, the IHP was positioned medial to the blood vessels in all cadavers. Therefore, to reach the pelvic organs, some of the arteries have to penetrate the IHP. In 33% of hemipelves (31/94), the inferior vesical artery, in 23% (22/94) the middle rectal artery, and in 6% of hemipelves (6/94) the superior vesical artery penetrated the IHP (Fig. 4). In 45% of female hemipelves (22/49), the uterine artery penetrated the IHP close to where it crossed the ureter (Fig. 5B). 124 Baader and Herrmann Fig. 4. Sagittal arrangement of the IHP and vessels in the pelvis. Shown is an adult male pelvis in a lateral view from the right side. The arrangement represents an AVN (artery-vein-nerve) layering showing the inferior vesical artery (3) most lateral followed by the pelvic veins (2) and the IHP (5). The middle rectal artery (4) deriving from the internal iliac artery (1) leaves the arterial layer to penetrate the IHP and to course toward the inferior part of the rectum. Orientation icons: sup, superior; inf, inferior; ant, anterior; post, posterior. Rectum The urinary bladder was observed to be innervated by the IHP in 79% of hemipelves (74/94). In general, all fibers coursed from a lateral, inferior, and posterior direction (Fig. 6A) such that fibers of the IHP were found lateral to the neck of the bladder and along the inferior vesical artery and ureter. The anterior surface of the urinary bladder was free of innervating fibers in all of the dissections in which they were observed. In half of the hemipelves investigated (47/94), the rectum was observed to be innervated by the IHP. The posterior part of the IHP innervated the superior part of the rectum; the inferior part of the rectum was innervated by inferior branches of the anterior part of the IHP. In addition, fibers accompanying the middle rectal artery after its penetration of the IHP provided innervation to the rectum. Of surgical importance is the finding that the rectum was always innervated on its lateral aspect, but never on its posterior aspect. Urinary System In 77% of hemipelves (72/94), a close association of autonomic fibers to the ureter could be shown. Irrespective of gender, only a few autonomic fibers coursed alongside the proximal part of the ureter within the abdomen and into the pelvis. During their course to the urinary bladder, the ureter and these autonomic fibers descended in parallel, and, just before the ureter entered the urinary bladder (near the seminal gland in males and near the isthmus of the uterus in females), the fibers of the IHP joined the ureter (Figs. 5A and 6A). Gender-Specific Organs An autonomic supply of the uterus was found in 73% of female hemipelves (36/49). Most of the fibers left the central portion of the anterior part of the IHP at about the isthmus of the uterus and innervated the uterus from a lateral direction (Fig. 5A,B). An additional supply could be shown from fibers running along the uterine artery as well as from fibers leaving the ureter as it crossed the uterine artery. The upper third of the lateral wall of the vagina was supplied by uterine branches of the IHP in 24% of cases (12/49); this might indicate a high degree of variability in the autonomic innervation of the vagina. Similar to the urinary bladder, the prostate was supplied by autonomic fibers from a lateral, inferior, Topography of Pelvic Autonomic Nervous System Fig. 5. Innervation of pelvic viscera by the IHP in fetal (A) and adult (B) females. A: Lateral view of the fetal visceral bloc from the right showing the ureter (1), IHP (2), cervix uteri (3), neck of the bladder (4), pudendal nerve (5), pelvic splanchnic nerve (6), and right 125 hypogastric nerve (7). B: Lateral view of the adult pelvic viscera from the left showing the uterine artery (1), internal iliac artery (2), IHP (3), uterus (4), and uterine tube (5). Orientation icons: sup, superior; inf, inferior; ant, anterior; post, posterior. 126 Baader and Herrmann Fig. 6. Innervation of pelvic viscera by the IHP in fetal (A) and adult (B) males. A: Lateral view of the fetal visceral bloc, right side. IHP (1), bladder (2), ureter (3), prostate (4), neurovascular bundle (5). B: Lateroposterior view of adult male pelvic viscera, left side. Poste- riorly reflected ureter (1) and ductus deferens (2), seminal gland (3), IHP (4), and urinary bladder (5). The IHP was reflected anteriorly by the hemostat. Orientation icons: sup, superior; inf, inferior; ant, anterior; post, posterior. and posterior direction (Fig. 6A), and its anterior surface was also free of innervating fibers. The innervation to the prostate was observed in 73% of male hemipelves (33/45). In about half of male hemipelves (23/45), the seminal gland was found to be innervated by the IHP; innervation was from all sides, but favored a lateral direction. Figure 6B illustrates the close relationship between the seminal gland and the IHP. In 40% of hemipelves (18/45), the ductus deferens was observed to be supplied by the IHP in its most distal part (Fig. 6B); innervating fibers arose from branches to the seminal gland. In the male fetus the ductus deferens was not surrounded by any autonomic nerves, suggesting that it obtains its innervation from the IHP secondarily during maturation. Neurovascular Bundle Besides the innervation pattern of the pelvic organs described above, the IHP is responsible for erectile function. The nervous stimulus for erection is provided by the cavernous nerves, which produce an increase in blood flow in the arteries that supply erectile tissue. The cavernous nerves derive from the most inferior part of the IHP and pass through the urogenital diaphragm along vessels, giving the combined structure the name “neurovascular bundle.” In males, Topography of Pelvic Autonomic Nervous System this neurovascular bundle was demonstrated in 40% of hemipelves (18/45). It formed at the inferior border of the prostate and penetrated the urogenital diaphragm parallel to the urethra (Fig. 6A). The pathway of the neurovascular bundle was found to be similar in females. It originated along the lateral surface of the vagina and traveled within the vesicovaginal septum and inferiorly through the urogenital diaphragm (Fig. 1B). The cavernous nerves in the female, however, were demonstrated in only 25% of hemipelves (12/49). DISCUSSION Topographic Aspects of the Inferior Hypogastric Plexus This study describes in detail the topography of the IHP that may be useful in improving nerve-sparing surgical approaches in pelvic surgery. The observations of the sympathetic and parasympathetic sources of the IHP described in previous reports were verified in our study; however, we expanded upon these studies by presenting a systematic view of the sources and distribution of fibers to and from the IHP. Also, previous reports described the sympathetic sources of the IHP as deriving from sympathetic ganglia S1 to S5 (Davis, 1933; Curtis et al., 1942; Pearson and Sauter, 1970; Walsh and Donker, 1982) and parasympathetic sources from S2 to S5 (Baljet and Drukker, 1981; Walsh and Donker, 1982; Stelzner et al., 1989). We demonstrated a more specific distribution in the participation of different ganglia and ventral rami. For example, sacral splanchnic nerve branches from sacral sympathetic ganglion S2 were the main sources of sympathetic fibers to the IHP and sacral ventral rami S3 and S4 were the primary sources of parasympathetic fibers to the IHP. Variations in results may be due in large measure to the fact that the autonomic nerve fibers are extremely tiny and difficult to dissect. An interesting observation of this study was that we demonstrated a similar source for the pudendal nerve and parasympathetic fibers forming the pelvic splanchnic nerves, suggesting that the somatic and autonomic nervous systems may be interrelated. Such a relationship was suggested earlier based on findings that the sacral ventral rami from which the autonomic and somatic fibers emerge have been identical (Clara, 1953; Alverdes, 1968) and that autonomic fibers were found to be enclosed within the pudendal nerve (Walsh and Donker, 1982; Colombel et al., 1999). A major focus of this study, the description of the topographic anatomy of the IHP within the pelvic cavity, is of critical significance in pelvic surgery. Basic aspects of the topography of the IHP along the hori- 127 zontal and coronal planes have been described previously in fetuses (Fritsch, 1989) and are consistent with the present findings. Extending previous work about the mediolateral organization of nervous and vascular structures in the pelvic cavity, we described more precisely the relationship of the nervous and vascular layers and the variety of their arrangements. The medial position of the IHP has significant implications for surgical procedures, as described in more detail below. We observed that the IHP is a homogeneously arranged ganglionic plate that is not divided into distinct layers and secondary structures on the macroscopic level. This is consistent with the findings of Baljet and Drukker (1981) and Lee et al. (1973). Although one can get the impression that trilaminar or bilaminar sheets exist within the IHP, as postulated by Ashley and Anson (1946), it is difficult to impose any functional significance to these layers. In addition, the frequently cited existence of multiple, widely separated subplexuses is unlikely. Even if there are distinct groups of neural crest cells responsible for the innervation of different organs (Kuntz, 1952), there is no data to suggest that they are distinguishable under intraoperative conditions. The autonomic innervation of the female genital tract, principally described by Frankenhäuser (1867), is consistent with the present findings. The uterosacral ligament, however, which is generally considered as containing only connective tissue, actually contains nerve fibers that travel to the uterus. A new finding of this study was the observation that the cavernous nerves in the female derived from the most inferior branches of the IHP and not from terminal branches of the vaginal plexus as postulated earlier (Testut, 1911). In contrast to the female cavernous nerves, the neurovascular bundles in males have been described previously in detail because of their importance in prostatic cancer surgery (Lue et al., 1984); this description is consistent with the present findings. Similarly, the innervation of the prostate is well known (Lepor et al., 1985; Schlegel and Walsh, 1987) and concordant with our study. In contrast to published literature describing no clear pattern of innervation of the seminal gland, it was found to be heavily surrounded by a network of IHP fibers in our study. The innervation of the urinary bladder is controversial. It has been reported that the bladder receives innervation from all sides (Ashley and Anson, 1946). It has also been demonstrated that the bladder receives only fibers traveling along with the ureter (Fritsch, 1989). Although there is little doubt that the ureter is accompanied by autonomic fibers (Davis, 1933; Stelzner, 1977), our study demonstrates an autonomic 128 Baader and Herrmann nerve supply to the bladder via direct fibers from the IHP from a lateral, inferior, and posterior direction as well as from indirect fibers traveling along the ureter and inferior vesical artery. This dual supply of the urinary bladder is similar to the innervation of the rectum. Whereas Long and Bernstein (1959) reported contributions from the IHP via fibers along the middle rectal artery, Fritsch (1989) reported a direct innervation of the rectum by the IHP from an anterolateral direction. Our observations support both of these pathways. A still-unanswered question remains: Are there autonomic fibers from the IHP to the posterior aspect of the rectum? Whereas Pearson and Sauter (1970) and Taguchi et al. (1999) described such an innervation pattern, we could not verify these findings. Implications of the Topographic Anatomic Knowledge of IHP on Surgical Interventions From our knowledge of the detailed topography of the IHP, we can now better predict regions of iatrogenic injury to the autonomic nervous system during surgery within the pelvic cavity. In the following discussion, we describe endangered regions in a superiorto-inferior orientation, and then describe specific implications for urologists, surgeons, and gynecologists. The promontory is the most superior region where operative injuries may occur. Aortocaval lymph node dissections, colorectal surgeries, and promontory fixations are surgical interventions that can injure the superior hypogastric plexus. Proceeding caudally, the retrorectal space (the mesorectum in surgical vernacular) is an important area of potential harm to sources of the IHP. For example, during a mesorectal excision for rectal cancer, the superior hypogastric plexus as well as the more inferiorly located pelvic and sacral splanchnic nerves can be injured. Pelvic lymph node dissections around the internal iliac artery can damage the IHP itself; therefore, knowledge of the topography of the vasculonervous layers can be extremely helpful. Dissections along the middle rectal artery bear potential risks not only to the nerves supplying the rectum but also for the entire IHP because it is formed by its contributing nerves around this artery. The anterior part of the IHP is endangered during surgical interventions near the distal end of the ureter, the uterine or inferior vesical arteries, the seminal gland, and Denonvilliers’ fascia. During ligations of the bladder and rectal stalks, as well as during dissection of the uterosacral ligaments, attention should be paid to dissecting immediately adjacent to the organs to prevent injury to nerves. The deepest region of predictable injury is the area where the neurovascular bundle pierces the urogenital diaphragm. It might be of general importance in pelvic surgery to first dissect the superior hypogastric plexus, which is relatively constant in its position, and then proceed into the depth of the pelvis along the bilateral hypogastric nerves, and finally to the IHP. Awareness of the structure of the IHP might significantly improve outcomes in all fields of pelvic surgery. After prostatectomies, only 10% of patients are still potent; cystoprostatectomy leads to impotence in all patients (Walsh and Mostwin, 1984). These poor outcomes prompted urologists to develop plexus-preserving methods. Walsh and Donker (1982) described a nerve-sparing technique that prevents injury to the IHP by: 1) transecting the membranous part of the urethra very close to its muscular wall during apical dissection of the prostate, and 2) ligating the lateral vascular and nerve supply to the prostate (known as the lateral pedicle in surgical vernacular) close to the organ during lateral mobilization. Based on more detailed neuroanatomic studies, Lepor et al. (1985) suggested an anterior incision of the lateral pelvic fascia to preserve the IHP and recommended using the prostatovesical vessels as landmarks for identifying the neurovascular bundle. Schlegel and Walsh (1987) described the seminal gland as the most important landmark to avoid injury to the IHP when dissecting the posterior pedicle. Based on these methods, the percentage of potent patients after prostatectomy has been reported at 69%, without compromising removal of tumor (Walsh et al., 1987). Surgeons have also been prompted to develop a nerve-sparing technique for rectal surgery because of an incidence of sexual and urinary dysfunction in 40 –100% and 7–70% of patients, respectively; the technique is called PANP (pelvic autonomic nerve preservation). Hojo et al. (1991) and Sugihara et al. (1996) describe four to five degrees of preserving autonomic pelvic tissue, depending on the preoperative staging: Degree 1 entails complete preservation of the superior and inferior hypogastric plexuses, whereas Degrees 4 and 5 only preserve the nerves from the ventral ramus of S4 to the urinary bladder. The best results have been obtained with Degree 1 surgeries, yielding no urinary dysfunction; however, 70% of patients still have sexual dysfunction. To improve outcome, the general conclusion of this and other work is that for mesorectal dissections it is important to stay medially and to dissect the superior hypogastric plexus with its hypogastric nerves (Long and Bernstein, 1959; Weinstein and Roberts, 1977; Hida et al., 1999). Furthermore, rectal dissection close to the rectal fascia (Muntean, 1999), identification of the middle Topography of Pelvic Autonomic Nervous System rectal artery as a landmark for the IHP (Long and Bernstein, 1959), preservation of Denonvilliers’ fascia within the prerectal space, and protection of the rectourethral fascia (described as the rectourethral muscle by Stelzner et al., 1989) for sparing the neurovascular bundle during the perineal approach (Stelzner et al., 1989) are all techniques that help to preserve the IHP and its sources and branches. In the 1960s, the supravaginal hysterectomy was preferentially used to preserve the autonomic supply of the pelvic viscera and the integrity of the pelvic floor in benign gynecologic diseases; however, because of the risk of developing a carcinoma of the cervical stump, gynecologists abandoned this procedure. Semm (1991, 1993) rediscovered the supracervical method using the intrafascial hysterectomy. This technique leaves a highly vascularized fascial collar and its contained nerves untouched, and enucleates the intrafascial cervical tissue, thereby eliminating a potential malignancy. Urinary and sexual dysfunctions could thus be prevented, and the vagina and pelvic floor remain intact, which seems to be of significant importance for female vita sexualis (Semm, 1991, 1993; Lüttges et al., 1994; Mettler et al., 1995). More serious problems in gynecology are oncologic interventions that fail to consider nerve-sparing techniques. Höckel et al. (1998), however, described a fascinating technique for cervical carcinoma. Their nerve-sparing, extended radical hysterectomy uses liposuction to remove all parametrial lymphoid tissue and subperitoneal adipose tissue without affecting the pelvic splanchnic nerves and the IHP. Additionally, they incised the vesico-uterine and recto-uterine pouches in the midline to ligate the uterine artery immediately after it branches from the internal iliac artery, to expose the IHP and its branches, and to dissect the recto-uterine and recto-vaginal ligaments in the anterolateral mesorectal planes. In conclusion, preserving the autonomic nervous system in the true pelvis has already been realized by the aforementioned surgical techniques and is currently a focus in several surgical fields. The techniques used, however, still need to be improved. This will require establishing a standard in surgery based on the topographic anatomy of the IHP, including its contributing nerves and branches. The present work demonstrates that the autonomic nerves of the pelvis can be dissected in detail at the macroscopical level; this should help the surgeon visualize anatomic relationships in the pelvis for further improving their nerve-sparing techniques. 129 ACKNOWLEDGMENTS The authors gratefully acknowledge the excellent technical assistance of Mrs. S. Doll and Mr. E. Voigt. REFERENCES Alverdes K, editor. 1968. Vegetatives Nervensystem. Leipzig: Edition Leipzig. p 712–724. Ashley FL, Anson BJ. 1946. The pelvic autonomic nerves in the male. Surg Gynecol Obstet 82:598 – 608. Baljet B, Drukker J. 1981. Some aspects of the innervation of the abdominal and pelvic organs in the human female fetus. Acta Anat 111:222–230. Clara M, editor. 1953. Das Nervensystem des Menschen. Leipzig: Barth. p 241–248. Colombel M, Droupy S, Paradis V, Lassau JP, Benoit G. 1999. Caverno-pudendal nervous communicating branches in the penile hilum. Surg Radiol Anat 21:273–276. Curtis AH, Anson BJ, Ashley FL, Jones T. 1942. The anatomy of the pelvic autonomic nerves in relation to gynecology. Surg Gynecol Obstet 75:743–750. Davis AA. 1933. The innervation of the uterus. J Obstet Gynaecol 40:481–507. Fraukenhäuser F. 1867. Dio nerven der Gebärmutter und ihre Endigung in den glatten Moskelfasern. Jena: Mauke. p III. Fritsch H. 1989. Topography of the pelvic autonomic nerves in human fetuses between 21–29 weeks of gestation. Anat Embryol 180:57– 64. Hida J, Yasutomi M, Maruyama T, Uchida T, Nakajima A, Wakano T, Tokoro T, Kubo R. 1999. High ligation of the inferior mesenteric artery with hypogastric nerve preservation in rectal cancer surgery. Surg Today 29:482– 483. Höckel M, Konerding MA, Heussel CP. 1998. Liposuctionassisted nerve-sparing extended radical hysterectomy: oncologic rationale, surgical anatomy, and feasibility study. Am J Obstet Gynecol 178:971–976. Hojo K, Vernava AM, Sugihara K, Katumata K. 1991. Preservation of urine voiding and sexual function after rectal cancer surgery. Dis Colon Rectum 34:532–539. Kuntz A. 1952. Origin and early development of the pelvic neural plexuses. J Comp Neurol 96:345–357. Lee JF, Maurer VM, Block GE. 1973. Anatomic relations of pelvic autonomic nerves to pelvic operations. Arch Surg 107:324 –328. Lepor H, Gregerman M, Crosby R, Mostofi FK, Walsh PC. 1985. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol 133:207–212. Long DM, Bernstein WC. 1959. Sexual dysfunction as a complication of abdominoperineal resection of the rectum in the male: an anatomic and physiologic study. Dis Colon Rectum 2:540 –548. Lue TF, Zeineh SJ, Schmidt RA, Tanagho EA. 1984. Neuroanatomy of penile erection: its relevance to iatrogenic impotence. J Urol 131:273–280. Lüttges JE, Lehmann-Willenbrook E, Semm K. 1994. Histopathological aspects of pelviscopic hysterectomy. Gynecol Obstet Invest 37:118 –122. Mettler L, Semm K, Lehmann-Willenbrock L, Shah A, Shah P, Sharma R. 1995. Comparative evaluation of classical in- 130 Baader and Herrmann trafascial-supracervical hysterectomy (CISH) with transuterine mucosal resection as performed by pelviscopy and laparotomy— our first 200 cases. Surg Endosc 9:418 – 423. Muntean V. 1999. The surgical anatomy of the fasciae and the fascial spaces related to the rectum. Surg Radiol Anat 21: 319 –324. Pearson AA, Sauter RW. 1970. Nerve contributions to the pelvic plexus and the umbilical cord. Am J Anat 128:485– 498. Schlegel PN, Walsh PC. 1987. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol 138:1402–1406. Semm K. 1991. Hysterektomie per laparotomiam oder per pelviskopiam. Geburtshilfe Frauenheilkd 51:996 –1003. Semm K. 1993. Intrafasziale vaginale Hysterektomie (IVH) mit oder ohne pelviskopischer Assistenz Geburtshilfe Frauenheilkd 53:873– 878. Stelzner F. 1977. Ueber Potenzstörungen nach Amputation und Kontinenzresektion des Rektums. Zentralbl Chir 102: 212–219. Stelzner F, Fritsch H, Fleischhauer K. 1989. Die chirurgische Anatomie der Genitalnerven des Mannes und ihre Schonung bei der Excision des Rectums. Chirurg 60:228 –234. Sugihara K, Moriya Y, Akasu T, Fujita S. 1996. Pelvic autonomic nerve preservation for patients with rectal carcinoma. Cancer 78:1871–1880. Taguchi K, Tsukamoto T, Murakami G. 1999. Anatomical studies of the autonomic nervous system in the human pelvis by the whole-mount staining method: left–right communicating nerves between bilateral pelvic plexuses. J Urol 161:320 –325. Testut L, editor. 1911. Traité D’Anatomie Humaine. Paris: Doin. p 276 –282. Walsh PC, Donker PJ. 1982. Impotence following radical prostatectomy: Insight into etiology and prevention. J Urol 128: 492– 497. Walsh PC, Epstein JI, Lowe FC. 1987. Potency following radical prostatectomy with wide unilateral excision of the neurovascular bundle. J Urol 138:823– 827. Walsh PC, Mostwin JL. 1984. Radical prostatectomy and cystoprostatectomy with preservation of potency. Results using a new nerve-sparing technique. Br J Urol 56:694 – 697. Weinstein M, Roberts M. 1977. Sexual potency following surgery for rectal carcinoma: a follow-up of 44 patients. Ann Surg 185:295–300.