* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1° infection
Survey
Document related concepts
Epidemiology of HIV/AIDS wikipedia , lookup
Plasmodium falciparum wikipedia , lookup
West Nile fever wikipedia , lookup
Neonatal infection wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Schistosomiasis wikipedia , lookup
Diagnosis of HIV/AIDS wikipedia , lookup
Marburg virus disease wikipedia , lookup
Leptospirosis wikipedia , lookup
Trichinosis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Hepatitis C wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Transcript
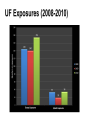
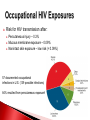
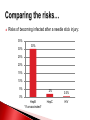
Biological Safety Office Environmental Health & Safety 352-392-1591 www.ehs.ufl.edu [email protected] Sharon Judge, PhD Assistant Biosafety Officer Pathogenic microorganisms present in blood and other potentially infectious material (OPIM) that are able to cause disease in humans Hepatitis B virus (HBV, HepB) Hepatitis C virus (HCV, HepC) Human immunodeficiency virus (HIV) Less commonly, human T-lymphotropic virus (HTLV-1), Epstein-Barr virus (EBV), malaria, brucellosis, rabies, leptospirosis, babesiosis, syphilis, Creutzfeld-Jakob disease, arboviral infections (WNV, EEE), etc. Implemented in 1991 by the Occupational Safety & Health Administration (OSHA) 29 CFR 1910.1030 http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051 Revised in 2001 – safe sharps devices, maintain a log of injuries from contaminated sharps UF follows OSHA requirement General and workplace-specific training Completed BEFORE individual is assigned to tasks with the potential for BBP exposure and ANNUALLY thereafter In addition to training, individuals with potential exposure must also have: Access to the regulatory text and an explanation of its contents http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051 Access to a copy of the UF Exposure Control Plan http://www.ehs.ufl.edu/Bio/BBP/ECP2011.pdf Access to site-specific Standard Operating Procedures (SOPs) http://www.ehs.ufl.edu/Bio/BBP/BBPSOPS.pdf Chairs/Directors Ensure dept. compliance Faculty/Supervisors Ensure appropriate exposure control plan is in place and being followed Employees, students, volunteers, etc Follow exposure control plan, report problems/exposure SHCC/Occ. Med Immunizations & post-exposure follow-up EH&S Biosafety Develop/coordinate program, track participants ALL employees, staff, students, volunteers, affiliates with potential exposure to BBP from human blood/OPIM Custodians, medical providers, dentists/dental staff, autopsy staff, clinical laboratory staff, research lab staff & students, biomedical engineers, athletic trainers, event staff, police, emergency responders, physical plant workers…etc YES NO* Cerebrospinal fluid Tears Synovial fluid Feces Peritoneal fluid Urine Pericardial fluid Saliva Pleural fluid Nasal secretions Semen/Vaginal secretions Sputum Breast milk Sweat Amniotic fluid Vomit *unless visibly contaminated with blood 1. Cuts or punctures with contaminated sharp objects 2. Splashes to mucous membranes 3. Contamination of broken/non-intact skin A woman in KY was arrested and charged with public intoxication (March 2010) While changing into an inmate uniform, she squirted a stream of breast milk into the face of a female deputy The press release sparked a debate when it was noted that the deputy was able to “clean the biohazard off of her” Does this constitute an occupational exposure? Yes, breast milk is considered OPIM All human blood or OPIM is treated as infectious Use: Safety Equipment Safe Work Practices Personal Protective Equipment (PPE) Standard precautions = universal precautions + body substance isolation. Applies to blood & all other body fluids, secretions, excretions (except sweat), nonintact skin, and mucous membranes Human blood and OPIM Objects/items contaminated by blood or OPIM Unfixed human tissues/organs (other than intact skin) Cell or tissue cultures that may contain BBP agents Blood/tissues from animals infected with BBP agents Use Universal Precautions for all human cell lines ATCC started testing newly manufactured/deposited cell lines for common viral pathogens (HIV, HepB, HepC, HPV, EBV, and CMV) in January 2010 Many infectious agents yet to be discovered and for which there is no test Remember HIV? What about XMRV? Spread through direct contact with infected fluids (blood, semen, vaginal fluids) Infection may be acute or chronic body ~4.3-5.6% of Americans have been infected with HepB 5-10 % of adults will develop chronic infection; ~1.2 million people with chronic HBV 15-25% develop cirrhosis , liver failure, or liver cancer (~ 3000 deaths/year) Many people (~50%) are asymptomatic; if symptoms occur they include: Fever Abdominal pain Fatigue Loss of appetite Nausea Vomiting Jaundice Joint pain Dark urine Percutaneous ~30% of these exposures results in infection Mucosal exposure to blood/body fluids Exposure to nonintact skin from contaminated surfaces and equipment – HBV can remain infective in dried blood at RT for at least 1 week (MacCannell et al., Clin Liver Dis 2010; 14:23-36) Get vaccinated! Universal Precautions Cleaning/disinfection Safe Effective Given to newborns, 120 million people in U.S. have received at least one dose >95% develop immunity after full series (3 doses given at 0, 1, 6 mos) In Gainesville, free @UF SHCC (392-0627) Bring completed Acceptance/Declination statement with you http://www.ehs.ufl.edu/Bio/BBP/TNV.pdf If you decline, can change mind at any time Health-care workers or public safety workers at high risk for continued percutaneous or mucosal exposure to blood or body fluids, HBV research lab workers Performed 1-2 months after dose #3 HepB surface antibody (anti-HBs) ≥ 10 mIU/mL - immune Anti-HBs < 10 mIU/mL – revaccinate (3 doses) and retest anti-HBs Still negative – nonresponder, need HBIG after exposure Previously vaccinated but not tested? Test for anti-HBs after an exposure; if negative, treat as susceptible. Transmitted primarily through contact with infected blood Many people asymptomatic (symptoms similar to HepB) ~1.8 % of Americans have been infected with HepC, 3.2 million chronically infected ~ 12,000 deaths/year Leading indication for liver transplant in U.S. Percutaneous injury, esp. with deep punctures or extensive blood exposures ~2% develop infection Mucosal/nonintact skin exposures rarely documented Proper cleaning/disinfection of surfaces important HCV in dried blood samples remains infective for at least 16 hours (Kamili et al., Infect Control Hosp Epidemiol 2007; 28:519-524) Universal Precautions for Prevention! NO VACCINE Antivirals (interferon/ribavirin) can have serious side effects, treatment lasts 24-48 weeks Transmitted through contact with infected blood/OPIM 1° infection Asymptomatic phase Symptomatic phase transient, non-specific illness (fever, malaise, muscle pain, sore throat) ↑ susceptibility to opportunistic infections, nonspecific constitutional symptoms (night sweats, weight loss, anorexia, fever) Advanced (AIDS) one or more opportunistic infections, CD4<200 cells/µl > 1 million living with HIV/AIDS ~56,000 new infections/year ~20% don’t know they are infected Florida ranks 3rd among states in the number of reported HIV/AIDS cases Risk for HIV transmission after: Percutaneous injury – 0.3% Mucous membrane exposure – 0.09% Nonintact skin exposure – low risk (< 0.09%) 57 documented occupational infections in U.S. (139 possible infections) 84% resulted from percutaneous exposure! No cure No vaccine Antiretroviral therapy – cocktail of 3 or more drugs, costly, side effects, drug resistance Always use Universal Precautions! Risks of becoming infected after a needle stick injury: 35% 30% 30% 25% 20% 15% 10% 5% 2% 0.3% 0% HepB *If unvaccinated* HepC HIV Engineering (safety equipment) ◦ Safety needles, sharps box, biosafety cabinet Work Practices ◦ Cleaning work surfaces, not recapping needles Personal Protective Equipment (PPE) ◦ Gloves, lab coat, face shield Maximum protection when these controls overlap Sharps container Biosafety cabinet Cleanable work surfaces/chairs Leak-proof transport containers Safety needles/syringes List of safety sharps devices available can be found at: http://www.healthsystem.virginia.edu/internet/epinet/safetydevice.cfm#1 Know what they are and follow them! Minimize splashes Don’t recap needles Know how to handle spills Wash your hands! No eating/drinking in areas where blood/OPIM is handled or stored NO!! NO!! Discard needles directly into sharps container Do not overfill the sharps box – close and replace when ¾ full Never attempt to re-open a closed sharps box Circumstances Associated with Hollow-Bore Needle Injuries NaSH June 1995—December 2003 (n=10,239) 35% disposal related FRESHLY DILUTED (w/in 24 hrs) 1:10 solution of household bleach EPA listed tuberculocidal disinfectant ◦ http://www.epa.gov/oppad001/chemregindex.htm ◦ Clorox, amphyl, lysol, sporicidin Ethanol evaporates too quickly to be an effective disinfectant! Notify people in the area 2. Don appropriate PPE (gloves, safety glasses) 3. Place absorbent material on spill 4. Apply appropriate disinfectant – allow sufficient contact time (30 min) 5. Pick up material (watch for glass – use tongs or dust pan); dispose of as biowaste 6. Reapply disinfectant and wipe For large/problematic spills, call EH&S Biosafety Office (392-1591) 1. Container of undiluted household bleach Several pairs of gloves Safety glasses Absorbent material Biohazardous waste (autoclave) bags Dust pan & scoop or tongs for broken glass Place in a labeled bag or bucket and keep in areas where biohazards are used Pay attention to frequently missed areas – fingertips, between fingers, under jewelry Wash hands after removing gloves & before leaving the work area If no sink nearby, use hand sanitizer and then wash with soap and water ASAP Wear it WHEN and WHERE you are supposed to PPE should never be worn in common areas (offices, hallways, bathrooms, cafeterias, etc) or when handling common-use items (doorknobs, elevator buttons, telephones) It is also common courtesy – others don’t know what you may have touched/where you have been PPE must be supplied by the employer It must fit, be suitable to the task (use common sense), and cleaned or disposed of properly (this does not mean taking it home to wash!) ◦ Gloves ◦ Face and Eye Protection Surgical mask, goggles, glasses w/side shield, face shield ◦ Body Gowns, aprons, lab coats, shoe covers Absolutely no open toed shoes in the lab! Gloves Never re-use or wash gloves! Some chemicals may breakdown the glove – use glove compatibility chart http://www.ehs.ufl.edu/Lab/CHP/gloves.htm Pay attention to how you remove your gloves! WASH HANDS! Site-specific! Equipment, practices, and PPE used AT YOUR SITE to protect you and others Written down, reviewed, accessible, updated annually or as needed Template for SOPs: http://www.ehs.ufl.edu/Bio/BBP/BBPSOPS.pdf More stringent control measures Work must be registered with EH&S Biosafety Office (rDNA or BA registration) Enrollment in medical surveillance program Follow CDC/NIH BSL-2 containment practices at a minimum Wash wound with soap & water for 5 minutes; flush mucous membranes for 15 minutes Seek immediate medical attention (1-2 hrs max) ◦ In Gainesville, call 1-866-477-6824 (Needle Stick Hotline) ◦ In Jacksonville, 7am-4pm, go to Employee Health Suite 505 in Tower 1; Other hours, go to ER ◦ Other areas, go to the nearest medical facility Notify supervisor Contact UF Worker’s Compensation Office, 352-392-4940 Allow medical to follow-up with appropriate testing & required written opinion Type/amount of fluid/tissue Infectious status of source Susceptibility of exposed person Percutaneous injury (depth, extent, device) Blood Presence of HepB surface antigen (HBsAg) and HepB e antigen (HBeAg) HepB vaccine and vaccine response status Mucous membrane exposure Fluids containing blood Presence of HepC antibody Immune status Type of exposure Non-intact skin exposure Presence of HIV antibody Bites resulting in blood exposure to either person CDC PEP Guidelines: http://www.cdc.gov/mmwr/PDF/rr/rr5409.pdf http://www.cdc.gov/mmwr/PDF/rr/rr5011.pdf Training records: ◦ Retain a minimum of 3 years Medical records for immunization or post-exposure follow up: ◦ Retain for duration of employment + 30 yrs (includes HepB vaccination records, vaccination declination statement) Confidential sharps injury log (type of device involved, where and how injury occurred): ◦ Retain for 5 years from date of exposure Warning labels must be placed on: ◦ Containers of regulated waste ◦ Refrigerators & freezers containing blood or OPIM ◦ Containers used to store, transport, or ship blood or OPIM Use red bags for waste containers