* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sedation in the Intensive Care Unit
Survey
Document related concepts
Transcript
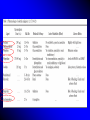
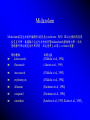
Sedation in the Intensive Care Unit: a general overview 台中榮民總醫院 內科部 加護中心 李博仁醫師 Current Forces in Critical Care • • • • • • • Institute of Medicine criteria for quality: Patient-centered: relevant outcomes define right care Effective: the right care Safe: the right care all the time Timely: the right care at the right time Efficient: the right care and only the right care Equitable: the right care for everyone 身體約束之合併症 •生理:約束超過4天感染率增加 皮膚撕裂傷、神經受損 肌力下降、血循減慢、便秘、失禁、 呼吸功能衰退甚至窒息或猝死 •心理:生氣、害怕 自主性及尊嚴受威脅— 惡夢 •社會:社交隔離 反應遲頓、社會功能變差 (David et al,2003) Death Caused by Physical Restrains Steven H.(1992) The Gerontologist •非計畫性拔管的盛行率8-13% •發生自拔管患者86.7%已接受約束 (游顯妹 ,2003) •非計畫性拔管的盛行率2-17% •身體約束在管路自拔的預防上是失敗的 (Gerald,2003) •美國食品藥物管理局(Food and Drug Administration) 表示每年有超過100位死亡或受傷的案例是因為照護 人員不當使用約束所造成 (Lusis, 2000) • 美國健康照護組織評鑑聯委會 約束:指限制個人在其環境中的活動自由或接近他 們自己身體自由度的過程。〈醫策會〉 醫院評鑑-約束醫囑及臨床照顧指引 身體約束:使用任何器具、材料或設備,將身體固 定,達到約束目的的方式。 化學性約束:使用精神用藥,限制非預期行為的產 生,達到約束目的的方式。 磁扣式腰腹約束衣 加強型手套式〈乒乓球〉約束帶 -台中榮總創 魚眼約束帶-台中榮總創 約束之執行過程 1.向病患或家屬詳細解釋約束的目的。 2.選用合適的約束用物。 (1)約束用物:如手腕或腳踝約束帶、約束衣、手套 式手腕約束帶等。 (2)約束時應避免壓迫動靜脈廔管、點滴注射處及其他引流管。 3.將叫人鈴放置在病患可觸及之處。 4.躁動患者每15分鐘應觀察約束部位血液循環情形、皮膚完整 性、關節活動狀況及身體擺位,評估有無合併症產生;一般 患者至少每4小時評估一次並記錄。 5.每2小時應解開約束帶一次,給予肢體活動或皮膚護理,協 助翻身或坐起。 6.每8小時重新評估約束的必要性 。 7.當約束的病患出現作嘔、不安等情形時,應立刻檢視病患有 無其他需求或潛在危險。 (Gerald,2003) 實施原則 1.持續評估病患狀況,儘可能運用身體約束替代措施。 2.當身體約束不需要時速解除約束。 3.約束用物應選用以最少的約束,提供最多的安全。 4.約束帶應以定位方式綁在床的骨架上,而不是床欄, 避免拉起或放下床欄時,拉扯約束帶。 5.綁約束帶應選用可迅速鬆開的方法。例如,綁成蝴蝶 結(綁在病人碰觸不到的地方 ),而不打死結。 6.作身體約束時,鬆緊應適中,保持1-2指之空隙,使 肢體能稍作移動。 7.約束時以腕關節約束為優先,且不可只約束踝關節, 以防病患自己鬆開約束或嘗試起來而跌倒。 Agitation & Stress hormone • Preexisting diseases (pancreatitis) • • Invasive procedures, or trauma.• • • Monitoring and therapeutic devices (such as catheters, drains, noninvasive ventilating • devices, endotracheal tubes) • Routine nursing care (such as airway suctioning ,physical therapy, dressing changes, and patient mobilization) • • Prolonged immobility Inadequate sleep Agitation Possibly causing exhaustion and disorientation. Evokes a stress response characterized by tachycardia, increased myocardial oxygen consumption, hypercoagulability, immunosuppression, and persistent catabolism NE ,Epi ,Glucogan ,ADH , Renin, Crotsol, Aldosterone, Serotonin, bradykinin, Prostagladin Pain assessment • Visual analogue scale (VAS) 視覺類比量表來評估病患之疼痛程度 • Numeric rating scale 0-10 數字等級量尺(0-10 numeric rating scale) (Geret al., 1999; Ger et al., 2004) • Behavioral-physiological scales • Family assessment • Verbal rating scale 疼痛分數1-4 分為輕度痛,5-6 分為中度痛,而7-10 分為重度痛 Pain rating scale 1. Simple descriptor scale 不痛,一點點,有些痛,很痛,痛極了 2. 0-10 numeric rating scale 0 1 2 3 4 5 6 7 不痛 8 9 10 痛極了 3. Visual analog scale (VAS) 痛極了 不痛 4. Faces rating scale .. .. .. .. .. .. .. ... Behavioral-physiological scale • Observation of pain-related behaviors – Movement, facial expression, posturing • Physiological indicators – HR, BP, RR How to do pain control • Set the goal and plan of analgesia • Opioid: fentanyl, hydromorphine, morphine • Scheduled opioid dose/ continuous IV better than “ as needed” • Hemodynamic instability, renal insufficiency: fentanyl, hydromorphine Patient-controlled analgesia vs conventional pain control A response from the past : Morphine (and its side-effects) Active metabolites: accumulation Constipation Respiratory depression 加護病房常使用的止痛劑性質及最小建議劑量 藥名 排除半衰期 Morphine 2-4 h Fentanyl 2-5 h 尖峰時間 30 min 4 min 最小建議劑量 1-4mg bolus 1-10mg/h infusion 25-100μg bolus 25-200μg/h infusion Hydromorphone 2-4 h Ketamine 2-3 h 20 min 0.2-1mg bolus 0.2-2mg/h infusion 30-60 sec 1-2μg/kg/min infusion Fentanyl patch • 強力的麻醉性止痛藥 (80-100xMorphine)過量中毒可致死 • 2005/7/15 FDA Issues Public Health Advisory • 呼吸困難、呼吸深度變淺、深度睡眠、無力正常思考、 無力正常說話及行走、有快昏倒的感覺、頭暈 • Fentanyl patch不應該使用於短期的疼痛、非持續的疼 痛、或手術後的疼痛。 Fentanyl patch只應該用於已經 使用過其他其他麻醉性止痛劑(對鴉片止痛劑opioids 具耐受性)的病患,還有使用短效的止痛劑卻無法有 效控制的慢性疼痛病患 FDA Public Health Advisory 2005/07/15 Delirium :an acutely changing or fluctuating mental status, inattention, disorganized thinking, and an altered level of consciousness Delirium and Critical Illness Brain Syndrome • Rates of delirium in non-critical care setting are around 10% to 20% • Rates of delirium in critical care settings are around 60% to 80% • Rates of acquired “dementia-like” critical illness brain syndrome following ICU care exceed 50% With an increasing proportion of inpatient critical care beds 1. Inouye et al, NEJM 1999;340:669-676 2. Ely et al, JAMA 2004;291-1753-1762 1.delirium was associated with a 3-fold higher rate of 1.Ely, Shintani, Speroff, JAMA 2003;289:2983-91 death by 6 months 2. 1.6-fold increase in ICU costs, and 10-fold higher 2.Milbrandt, Crit Care Med 2004;32:955-962 rate of cognitive impairment at hospital discharge (p<0.001) Risk Factors, Prevention, and Treatment • • • • • • Aging Baseline dementia Underlying illness – Inflammation – Coagulation Metabolic disturbances • Hypoxemia • Genetic Predisposition • Psychoactive Medications • Sleep Deprivation Inouye, JAMA 1996;275:852-57 Dubois, Intens Care Med 2001;27:1297-1304 Inouye, NEJM 1999;340:669-676 Jacobi, Crit Care Med 2002;30:119-141 Milbrandt, Crit Care Med. 2005;33:226-9 1st prevent... 2nd treat ICU delirium • Treat underlying infection and CHF • Correct metabolic disturbances and hypoxemia • Goal-directed delivery of sedation/analgesia • Frequent reorientation of patient by nurse and family • Stop the ventilator each day to test readiness for liberation • Early mobilization and physical therapy • Attention to optimizing sleep patterns Haloperidol • • • • Commonly given via intermittent i.v. injection The optimal dose and regimen of haloperidol have not been well defined. Haloperidol has a long half-life (10–24 hours) and loading regimens are used to achieve a rapid response in acutely delirious patients IM、IV 2-5 mg loading, M= 5 mg /h • Eric Milbrandt ESICM 17th Annual Congress: Abstract 251. Presented Oct. 11, 2004 • Haloperidol Improves Survival in Mechanically Ventilated, Critically Ill Patients • Haloperidol has anti-inflammatory effects on cytokines by mean dose of 11.5 (± 11.6) mg/day for a mean period of 3.5 (± 4.6) days record 1,095 ICU patients during the past year that were mechanically ventilated for a period of longer than 48 hours Agitation • • • • • Patients factors Environmental People Drugs and devices Technology pain anxiety VO2 increase respiratory drive sleep disturbances Measures process (Ramsay scale) and communication 理想的鎮靜劑 • • • • • (l)對血液動力學或肺功能沒有不良影響 (2)重覆給藥時無毒性代謝物之產生或積聚 (3)不影響其它藥物之代謝 (4)藥物代謝途徑不依靠腎、肝、肺等器官 (5)短效並具高療效、便宜、不需複雜或昂貴的 配備 • (6)給藥途徑可經由靜注(持續性或間歇性)並 可依病人病情之需要隨時調整劑量 Goals of Analgo-Sedation Ability to tolerate physical enviroment Ability to tolerate ICU procedures Prevention/reduction of stress Patient safety Sedation-agitation scale-I • 7. Dangerous agitation – Pulling ET tube, cath, climbing over bed rail, striking at staffs, thrashing side to side • 6. Very agitated – Not calm, depite frequent verbal reminding of limits, requires physical restraints, bites ET tube • 5. Agitated – Anxious ormildly agitated, attempting to sit up, calms down to verbal instructions Sedation-agitation scale-II • 4. Calm and cooperative – Calm, awakens easily, follows commands • 3. Sedated – Difficult to arouse, awakens to verbal stimuli or gentle shaking but drifts off again, follows simple commands • 2. Very sedated – Arouses to physical stimuli but does not communicate or follow commands, may move spontaneously • 1. Unarousable – Minimal or no response to noxious stimuli, does not communicate or follow commands Ramsay Sedation Scale Level of sedation: 1. 2. 3. 4. Patient is anxious and agitated Patient is cooperative, oriented and tranquil Patient responds to command only Patient exhibits brisk response to light glabellar tap or loud auditory stimulus 5. Patient exhibits a sluggish response to light glabellar tap or loud auditory stimulus 6. No response to stimuli Why should we adopt sedation scoring? Objective assessment and close, prospective control of the level of sedation DeJonghe B et al: Using and understanding sedation scoring systems: A systematic review. Intensive Care Med 2000; 26: 275–285 Brook AD et al: Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med 1999; 27:2609–2615 Advantages of Sedation scales No risk of over-sedation and under- sedation Optimal end-point for titration of sedation Prospective management of care Comparability of drugs effects Over-sedation:drawbacks Respiratory depression Hypotension Bradycardia Venous stasis Increased lenght of ventilation Increased ICU lenght of stay Increased costs Failure to evaluate CNS alterations benzodiazepine Choride channel (closed) Cl¯ + + Empty receptor is inactive, and the coupled chloride channel is closed GABA receptor Receptor empty (no agonists) – – Benzodiazepine receptor GABA ++ Receptor binding GABA GABA CI¯ ++ GABA receptor –– Binding of GABA causes the chloride ion channel to open –– CI¯ Benzodiazepine +++ Receptor binding GABA and a benzodiazepine CI¯ GABA +++ GABA receptor ––– Entry of chloride ions hyperpolarizes the cell, making it more difficult to depolarize and therefore reducing neural excitability ––– CI¯ CI¯ CI¯ Binding of GABA is enhanced by the benzodiazepine, resulting in a greater entry of chloride ions lorazepam 2 mg bolus iv 2mg /h for 7 days Residual lorazepam effect > 3 days after discontinuation of the infusion • Lorazepam solvent: polyethtlene glycol (PEG), propylene glycol (PG) – Lactic acidosis – Acute tubular necrosis Midazolam Anterograde amnesia:會造成前進性記憶喪失,通常在手術及診 斷療程前或進行中時,非常有用 代謝(Metabolism) Midazolam在體內可完全且迅速被代謝。主要代謝物為-hydroxy-midazolam。 約有40-50%的劑量乃經肝臟代謝。 排泄(Elimination) 健康志願者之排除半衰期為1.5-2.5小時,血中廓清率為300-400公撮/分鐘。當 靜脈輸注midazolam,其排除動力學與bolus注射時並無不同。主要代謝物hydroxy-midazolam之排除半衰期較原成份短。該代謝物會與glucuronic acid 結合(無活性)並由腎臟排出體外 60歲以上成人之排除半衰期可能延長達三倍,而有些需以靜脈輸注 midazolam以達長效鎮靜之ICU患者,可能高達六倍。這些病患在不 改變輸注速率下,於穩定狀態時可得較高的血中濃度。 充血性心衰竭及肝功能降低(reduced hepatic function)的患者可能有較長 之排除半衰期。 Midazolam Midazolam與某些抑制肝臟酵素(特別是cytochrome P450 IIIA)之藥物間具潛 在交互作用。數據顯示這些化合物會影響midazolam的藥物動力學,而改 變鎮靜作用的程度及作用時間,因此使用上必須小心 titeate 劑量。 藥物種類 ketoconazole 相關文獻 (Olkkola et al., 1994) fluronazole (Ahonen et al., 1999) itraconazole (Olkkola et al., 1993) erythromycin (Olkkola et al., 1994) diltiazem (Backman et al., 1994) verapamil (Backman et al., 1994) cimetidine (Sanders et al., 1993; Kanto et al., 1983), benzodiazepine (Pharmacology) 藥理作用 進行的試驗 效力比較 Anxiolytic effect Conflict test Valium : Dormicum = 1 : 1 Sedative effect Conflict test Valium : Dormicum = 1 : 2 Irritation threshold Anger reaction Valium : Dormicum = 1 : 2 Muscle relaxant Pole-climbing test Valium : Dormicum = 1 : 1 Anticonvulsant Standard test Valium : Dormicum = 1 : 1 Data from F. Hoffmann La Roche Ltd., Basel, Switzerland Anexate® (Flumazenil) • Flumazenil限使用於: 全身麻醉病例 Benzodiazepin類藥物中毒之鑑別診斷與 治療病例。 • 【全民健康保險藥品使用規範】 加護中心常用的鎮靜劑 Drug Initial dosage Maintain dosage Midazolam 0.05-0.10mg/kg 0.1mg/kg/h Diazepam 0.10-0.20mg/kg 1-4mg/h Lorazepam 2-4mg 0.5-1mg/h Propofol 1-2mg/kg 1-3mg/kg/h Midazolam-Induced Sedation for Upper Gastrointestinal Endoscopy: Assessment of Endoscopist and Patient Satisfaction Anterograde amnesia • 352 patients upper gastrointestinal endoscopy were sedated with midazolam given • Ages of the patients ranged between 16 and 79 years (average: 41.6 ± 12.7 years). • Anterograde memory was found in 310 (88.0%) • 342 patients (98.0%) cooperated well • Side effects were rarely seen (3.6%), and included nausea, vertigo, and vomiting • Acceptability of further endoscopy in 338 (96.0%) • No significant cardiopulmonary problems Gastroenterology Nursing: Volume 26(4) July/August 2003 pp 164-167 Lorazepam vs Midazolam Infusion data Prospective randomized trial Time to achieve adequate initial sedation (min) Infusion rate at point of initial sedation (mg/kg/hr) Maximum infusion rate (mg/kg/hr) Mean infusion rate (mg/kg/hr) Total time of infusion (hr) Total drug administered (mg/kg) Lorazepam (n=10) Midazolam (n=10) p-value 124 168 105 101 NS 0.06 0.05 0.15 0.15 NS 0.1 0.06 0.29 0.20 0.006 0.06 0.04 0.24 0.16 0.004 77 66 108 60 NS 5.4 7.0 23.3 19.0 0.01 Pohlman A.S., et al. Crit Care Med 1994; 22: 1241-1247 Lorazepam vs Midazolam Time to achieve adequate sedation Time of neurologic recovery p= NS 4000 300 250 p= NS 3000 200 2000 150 100 1000 50 0 Lorazepam Midazolam 0 Lorazepam Midazolam Pohlman A.S., et al. Crit Care Med 1994; 22: 1241-1247 Propofol vs Midazolam Effectiveness of sedation n=97 First hour of treatment p= NS % Assessments 70 After first hour of treatment # 80 p< 0.01 70 60 60 50 50 40 40 30 30 20 20 10 10 0 0 Effective Acceptable Ineffective propofol # Effective Acceptable Ineffective midazolam Chamorro C., et al. Crit Care Med 1996; 24: 932-939 Propofol vs Midazolam Monitoring the patient state of sedation Prospective randomized multicenter trial n=97 80 p< 0.05 % Assessments 70 60 50 40 30 20 10 0 propofol Very easy Easy Moderate Difficult midazolam Chamorro C., et al. Crit Care Med 1996; 24: 932-939 Sedation in the general ICU Speed of recovery after sedation Patient type Evaluable patients (n) Duration of Recovery infusion endpoint Mean (range) (h) 81 88 Postsurgical, medical, trauma 21 18 20 21 Postsurgical (abdominal) 17 17 approx. 6 approx. 6 Midazolam 90 120 Maximum level of 23 137 consciousness Medical, trauma, 32 postsurgical, 18 miscellaneous ‘Diprivan’ Recovery time Mean (min) 0 30 60 (3-24) (4-47) Weaning from ventilator 5 148 Spontaneous ventilation 16.4 85.2 150 180 p<0.05 p<0.001 p<0.05 1. Chamorro C et al. 1996. 2. Aitkenhead C62 et al. 1989. 3. Wolfs C et al. 1991. Hemodynamic Effects of Midazolam and Propofol • • • • • • Parameter BP, 1st hr SBP MAP MAP Decr SBP Midazolam incr 21% decr 12% NC NC 31% pts Propofol decr 17% decr 24% decr 17% decr 33% 68% pts Comment p < 0.01 decr > 20% Ref 1 2 3 4 5 • Propofol (1-2.5 mg/kg bolus, then 3 mg/kg/h) decreased MAP by 6 43%, CI by 23%, SVRI by 30% • • • 1.. Boeke et al. J Drug Dev 1989 3. Kox et al. Br J Anaesth 1990 5. Weinbroum et al. ICM 1997 2. Geller et al. Anesthesiology 1991 4. Pappagallo et al. Minerva Anest 1992 6. Martin et al. Acta Anaesth Scand ?4 Sedation of the ICU patients during mechanical ventilation: propofol or midazolam? Propofol and midazolam achieved optimal sedation when administered by specified dosing protocol Propofol had a faster awakening time Time to sedation was not significantly different VO2 decreased similarly in both groups Propofol is the preferred sedative when rapid awakening (e.g., for neurologic assessment or extubation) is important. (Grade of recommendation B) Kress J., et al. AJRCCM 1996; 153: 1012-1018 • propofol did not produce amnesia as often as midazolam . Like the benzodiazepines, propofol has no analgesic properties. • Provides 1.1 kcal/mL from fat and should be counted as a caloric source. Long-term or high-dose infusions may result in hypertriglyceridemia • Pancreatitis has been reported following anesthesia • Prolonged use (48 hours) of high doses of propofol (66 g/kg/min infusion) has been associated with lactic acidosis, bradycardia, and lipidemia in pediatric patients -***FDA against the use for the prolonged sedation • incidence of infectious complications---no more than 12 hours Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult Crit Care Med 2002 Vol. 30, No. 1 Continuous IV Sedation and Duration of Mechanical Ventilation • Patients receiving continuous IV sedation: CIVS 20 – younger – lower PaO2/FIO2 – more ARDS * Days • more chemical paralysis • CIVS had longer LOSs after adjustment for age, SOI, mort, MV indication, paralysis, trach, # OSF No CIVS 10 * 0 MV duration ICU LOS Hosp LOS * p < 0.001, + p = 0.007 Kollef MH, et al. Chest 1998;114:541 Complications of Sedative Medications • Cardiovascular alterations • Respiratory depression, apnea • Prolonged sedation The titration of the sedative dose to a defined endpoint is recommended with systematic tapering of the dose or daily interruption with retitration to minimize prolonged sedative effects. (Grade of recommendation A) • Tolerance and tachyphylaxis • Withdrawal Sx • Drug-specific – Propofol: Increased triglycerides – Lorazepam: precipitation Short-term sedation (24-48 H) • Status asthmaticus • COPD with acute exacerbation, but without sepsis • Agitation due to mechanical ventilation without major sepsis • Delirium in ICU • Severe CAP, acute respiratory failure, unable to tolerate ET tube What drugs for short-term sedation • Midazolam 2-5mg iv until acute exacerbation event controlled • Check the indication of sedation on 2nd and 3rd day – Stop – Tappering – Switch to long-term sedation Midazolam is recommended for shortterm use (Grade of recommendation A) Long-term sedation (> 72 H) • Severe sepsis and septic shock • ARDS with refractory hypoxemia • Prone position What drugs for long-term sedation (1) • Midazolam 2-5mg iv q5~15 min until acute event controlled • Start continuous midazolam, titrated to the level of sedation • Check the sedation level everyday • Check the indication on 3rd and 4th day – Stop or tappering – Add lorazepam 1-2 amps q2-6 hours, tappering midazolam to the defined level of sedaiton What drugs for long-term sedation (2) • On 6-10th days – Tapering the lorazepam, depending on the indication of sedation – Restarting the midazolam or propofol to replace the role of lorazepam – Preparing the patient wake-up and weaning Summary Individualized approach to sedation Propofol is useful for deeper level of sedation and more rapid awakening Benzodiazepines should be used to provide rapid amnesia (midazolam) or long-term sedation (lorazepam) Randomized studies needed to investigate safety profile/costefficiency of new generation-opioids, that present promising aspects Dexmedetomidine Underused for Anesthesia, Sedation • The alpha-2 agonist is a sedative with analgesic and anxiolytic properties. It was granted FDA approval • SCCM 35th Critical Care Congress: Situational Sedation and Analgesia, presented January 9, 2006; abstract • Approximately 70% of the audience indicated that they never used the agent • used in combination with propofol, opioids, and anxiolytic agents Dexmedetomidine • respiratory stability and easy routine stability • slow the heart rate, the hemodynamic response is predictable • "there is no need to discontinue [the drug] prior to extubation." • No loading dose is required with the drug • High dosage cause significant bradycardia and hypotension • need to use caution when administering it to patients with hypovolemia or heart block • very expensive • more pain and discomfort and poorer sleep quality than other sedatives, such as propofol • dexmedetomidine in 39 children admitted to the pediatric intensive care unit (PICU) after heart surgery between October 2004 and June 2005 • ranged in age from 3 months to 18 years • 92% of the patients needed no supplemental medication with fentanyl, midazolam, or other sedative agents while receiving dexmedetomidine, • 79% received minimal or no supplemental analgesia • Reduction in systolic blood pressure was less than 8% and heart rate reduction was less than 12% in the first 4 hours • no appreciable hemodynamic changes • no evidence of respiratory depression • no cases of rebound or withdrawal after either weaning or abrupt discontinuation of the drug • Survival was 100%. 癱瘓敵軍 美考慮用肌肉鬆弛劑 • 藥物能夠「有效影響中樞神經系統組織, 並產生較不焦慮、較不具攻擊性,以及 較為鎮定的行為」 • 賓州州立大學科學家們推薦「立即考慮」 使用的藥物,包括diazepam,也就是肌 肉鬆弛劑煩寧的主要成分,以及用來鎮 定加護病患的dexmedetomidine 〔自由時報編譯中華民國91年5月27日星期一 〕