* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Foundation 1 - Discovering Astronomy
Survey
Document related concepts
Transcript
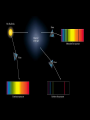
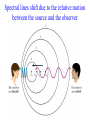
Chapter 4 The Origin and Nature of Light What do you think? • How hot is a “red hot” object compared to objects glowing with other colors? • What color is the Sun? What can we learn by analyzing starlight? • A star’s temperature Peak color (wavelength) shifts to shorter wavelengths as an objects is heated increasing temperature Peak color (wavelength) emitted depends on an object’s temperature Peak color (wavelength) shifts to shorter wavelengths as an objects is heated hotter object cooler object The intensities of different emitted colors reveal a star’s temperature • Wien’s law • wavelength is inversely proportional to temperature lmax = (2.9 x 10-3) / TKelvin wavelength is inversely proportional to temperature lmax = (2.9 x 10-3) / TKelvin What color is our 5800K Sun? The Sun emits all colors (wavelengths of electromagnetic radiation); however, the colors it emits most intensely are in the blue-green part of the spectrum. What can we learn by analyzing starlight? • A star’s temperature • A star’s chemical composition Each chemical element produces its own unique set of spectral lines when it burns The Sun’s Spectrum The brightness of spectral lines depend on conditions in the spectrum’s source Law 1 A hot object or a hot, dense gas produces a continuous spectrum -- a complete rainbow of colors with without any specific spectral lines. (This is a black body spectrum.) The brightness of spectral lines depend on conditions in the spectrum’s source Law 2 A hot, rarefied gas produces an emission line spectrum - a series of bright spectral lines against a dark background. The brightness of spectral lines depend on conditions in the spectrum’s source Law 3 A cool gas in front of a continuous source of light produces an absorption line spectrum - a series of dark spectral lines among the colors of the rainbow. Absorption Spectrum of Hydrogen Gas Kirchhoff’s Laws But, where does light actually come from? Light comes from the movement of electrons in atoms An atom consists of a small, dense nucleus surrounded by electrons Atomic Vocabulary • The nucleus contains protons and neutrons • All atoms with the same number of protons have the same name (called an element) • Atoms with varying numbers of neutrons are called isotopes. • Atoms with a varying numbers of electrons are called ions. Spectral lines occur when an electron jumps from one energy level to another What can we learn by analyzing starlight? • A star’s temperature • A star’s chemical composition • A star’s movement – Barnard’s Star Spectral lines shift due to the relative motion between the source and the observer Doppler Shifts • Red Shift: The distance between the observer and the source is increasing • Blue Shift: The distance between the observer and the source is decreasing The Doppler shift allows astronomers to measure radial velocity What can we learn by analyzing starlight? • A star’s temperature – by peak wavelength • A star’s chemical composition – by spectral analysis • A star’s radial velocity – from Doppler shifts What did you think? • How hot is a “red hot” object compared to objects glowing with other colors? Of all objects that glow from heat stored or generated inside them, those that glow red are the coolest. • What color is the Sun? The Sun emits all colors (wavelengths of electromagnetic radiation). The colors it emits most intensely are in the blue-green part of the spectrum. Self-Check 1: State the Stefan-Boltzmann law and Wien’s law and explain their meaning in the context of blackbody radiation and temperature determination. 2: Describe the evidence for the particle nature of light and indicate how the energy per photon is related to the wavelength and frequency in the wave model. 3: State Kirchhoff’s three laws of spectral analysis and indicate what information is derived about the nature of the light source in each case. 4: Describe the Bohr model of the atom in terms of its constituents and their distribution. Explain how spectral lines can be produced by a low-density gas. 5: Describe how spectroscopic analysis provides information about the chemical composition of celestial objects and indicate for which part of the object the information is valid. 6: Indicate how the numbers of protons, neutrons, and electrons are used to define elements, ions, and isotopes. 7: Describe the origin of line series in the hydrogen atom and explain why the Balmer lines occur at visual wavelengths but the other line series do not. 8: Define excitation and ionization in the context of the Bohr model of atoms. 9: Describe how the Doppler shift reveals the radial motion of the stars.