* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Transfusions in Surgery
Hemorheology wikipedia , lookup
Blood donation wikipedia , lookup
Men who have sex with men blood donor controversy wikipedia , lookup
Autotransfusion wikipedia , lookup
Blood transfusion wikipedia , lookup
Jehovah's Witnesses and blood transfusions wikipedia , lookup
Hemolytic-uremic syndrome wikipedia , lookup
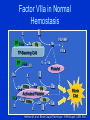
Transfusions in Surgery Dr. Carolyn Faught November 28, 2006 FACT • Approximately 60% of all red cell transfusions are administered to surgical patients • Therefore need to understand indications and risks of transfusion Main Objectives • Be aware of the adverse effects of transfusion • Discuss “transfusion triggers” in surgical patients • Treatment of massive transfusion • Indications for recombinant FVIIa in surgical patients Blood Components • Blood components made by physical separation from whole blood: – – – – Packed red blood cells (PRBCs) Platelets Plasma ( FFP, cryoprecipitate) Granulocytes • Blood derivatives: – Fractioned, virally inactivated product (ie. albumin, IVIG) – Recombinant products (VIII, IX, VIIa) Universal donor/ Universal recipient • Group O RBCs: universal donor, can be given to patients of all blood groups, because no A or B antigens on surface of RBCs • Group AB person is the universal recipient for RBCs, because has no anti-A or anti-B antibodies in plasma • Group AB plasma can be given to all recipients, as it has no anti-A or anti-B antibodies • Emergency (uncrossed) products = O neg RBCs and AB plasma • Still best to give Group specific blood Blood Products: Universal leukoreduction of all blood products in Canada • ‘third generation’ filters to remove WBCs from blood (leukoreduction) to less than 5x106 WBC/bag of RBCs • Leukoreduction reduces CMV transmission, HLA alloimmunization, febrile non-hemolytic transfusion reactions Adverse Effects of Transfusion • Transfusion Reactions – Infectious – Non-infectious • Immunologically mediated • Non-immunologic – Immediate – Delayed Infectious risks of transfusion • • • • • Bacterial contamination of platelets 1:40,000* HBV - 1/250,000 HIV - 1/1,900,000 HCV - 1/1,800,000 HTLV1 - 1/1,000,000 Less clear • CJD (Creutzfeldt-Jakob disease) • West Nile 1/4,000 Non-infectious risks • • • • • • Febrile reactions Allergic/anaphylactoid reactions Hemolytic reactions – 1/20,000 Volume overload Acute lung injury (TRALI) Serologic sensitization – Alloimmunization to red cell antigens (delayed hemolytic transfusion reaction) – Graft-versus host disease – Platelet refractoriness, post-transfusion purpura • Immunomodulation - ? Increased risk of infection, cancer recurrence TRALI • • • • Transfusion-Related Acute Lung Injury Non-cardiogenic pulmonary edema after transfusion Incidence up to 1:5000 transfusions Antibodies in donor plasma against HLA or neutrophils react with recipient leukocytes • Leukoagglutination in lungs, increase in vascular permeability, fluid enters alveolar spaces • Need to identify, to remove donor from donor pool TOH Blood Conservation Program • 800 patients a year assessed at Civic • Best candidates are hips, backs, prostate, some gynecologic surgery • Patients assessed 4 wks prior to surgery so they can benefit • Liberal use of Epo and iron • Autologous or 2 unit collection with TRIMA • If Hb > 130 g/L, no Epo • If Hb < 125-130 g/L, no autologous donation Transfusion Rates Across Canada • Locally, transfusion rates are ~ 45% in hips, 35% in prostate, 25% in knee • Across Canada: – Cardiac: 43.8% female 65% vs male 37% – Hip: 34.4% – ICU (post-op and trauma): 36.9% Can J Anesth 2005: 52(6); 581-590 Transfusion Practices in Surgery • Little evidence to support age-old practice of keeping Hb > 100g/L • General trend towards conservative RBC transfusion practices in elective circumstance • TRICC Trial largest RCT showed RBC transfusion threshold of 70 g/L was as safe as 100 g/L in terms of morbidity and mortality in the critical care setting Hebert, P.C., NEJM 340(6), 1999;409-418 Red blood cell transfusion practices amongst Canadian anesthesiologists: a survey • A threshold above 70 g/L chosen by 48% for general surgery, 56% for orthopedic surgery and 9% for vascular surgery • History of coronary artery disease associated with threshold of 100 g/L • Very young age associated with threshold of </= 60 g/L • Conclusion: general adoption of TRICC trial even in surgical patients Can J Anesth 2006: 53(4); P 344-352 Platelet storage Fresh Frozen Plasma (FFP) • Fresh Frozen Plasma contains the clotting factors necessary for the hemostatic process • Plasma also has volume expansion and oncotic properties • Typical adult dose = 10-20 ml/kg, 4-6 units • Unlikely to be beneficial if PT and/or aPTT <1.5x normal Massive Transfusion (MT) and Coagulopathy • Definition: replacement of one blood mass in a 24 hour period • More practical definition: transfusion of four or more PRBCs within one hour or replacement of 50% blood volume in three hours • Uncontrolled bleeding causes 40% of deaths in severe trauma Hemorrhage • Symptoms relate to amount lost • Blood volume (adult) = 75 ml/kg = 5 liters • 10% loss: few symptoms • 20% loss: postural hypotension • 40% loss: shock • 50% loss: irreversible shock Massive Transfusion and Coagulopathy • Hypothermia – slows activity of coagulation cascade, increases fibrinolysis and alters platelet function • Dilutional coagulopathy – exacerbated by initial volume contraction, fluid resuscitation and transfusion of factor and platelet-deficient PRBCs • Fibrinogen < 1.0 g/L when blood loss 142% • DIC The contribution of red cells to hemostasis Disseminated Intravascular Coagulation • Hemostatic defects related to the exaggerated generation of thrombin and fibrin and the excessive consumption of platelets and coagulation factors • Low incidence in elective surgery • In trauma patients: • Due to degree of tissue trauma and tissue anoxia • 40% incidence in brain injury, 25% without head injury Disseminated Intravascular Coagulation • Incidence of coagulopathy 98% if all of the following: pH less than 7.10 Temperature less than 34C SPB less than 70 mm Hg Injury severity score greater than 25 Ferrara A, J Trauma 1997;42:857-61 Management of Coagulopathy in MT • PT/PTT increase when factors V, VIII and IX < 50% • Both PT/PTT increased if fibrinogen is low • PT or PTT ratio ≥1.8 means factors are < 30% and leads to high rate of bleeding • No prophylactic regimen of FFP/platelets concentrates has been shown to be effective in MT Management of Coagulopathy in MT • Correct hypothermia • Correct low Hb – optimum Hb in MT to maintain hemostasis unknown but probably higher than required for O2 delivery • Correct markedly prolonged INR/PTT with FFP ( ratio > 1.5) • Typical use 4-6 units (800-1500 ml) in average adult or 10-20 ml/kg; – gross underestimate in severely depleted, actively bleeding, hypothermic patient and should be 1-1.5:1 ratio of FFP to PRBCs Management of Coagulopathy in MT • If fibrinogen < 1.0 g/L despite FFP administer cryoprecipitate: 10-20 units • Platelets for decreased platelet count with clinical coagulopathy • Consider rFVIIa if above not sufficient to control bleeding J.F.Hardy,Can J Anesth 2004;51(4):293-310 Recombinant factor VIIa (Niastase) Background • Recombinant factor VIIa developed to treat hemophilia patients with inhibitors – By-passing agent in patients not responding to factor replacement • Recombinant factor VIIa used for treatment and prophylaxis of hemophiliacs with inhibitors • Use expanded to other bleeding patients Factor VIIa in Normal Hemostasis II X TF VIIa Xa VIII/vWF Va IIa VIIIa TF-Bearing Cell TF VIIa IXa V IX X Va Platelet II Xa VIIIa Va IXa Activated Platelet VIIa Va IXa VIIIa Xa IX X II IIa Fibrin Clot IIa Hoffman M, et al. Blood Coagul Fibrinolysis. 1998;9(suppl 1):S61-S65. FVIIa – FVIII and FIX Independant II X TF VIIa Xa Va IIa TF-Bearing Cell TF V Va Platelet II X VIIa Xa Va Activated Platelet IIa Fibrin Clot Hoffman M, et al. Blood Coagul Fibrinolysis. 1998;9(suppl 1):S61-S65. High-Dose FVIIa Enhances IIa Generation Without TF Relative IIa generation 4 Normal Hemophilic 3 2 1 0 0 1 10 100 [FVIIa] (nM) Monroe DM, et al. Br J Haematol 1997;99:542-547. Niastase: Licensed Indications • Treatment of bleeding in hemophilia patients with inhibitors • Surgical management of hemophilia patients with inhibitors • Congenital factor VII deficiency (USA) • Glanzmann’s Thrombasthenia (UK) Pharmacology of Recombinant Factor VIIa • 50,000 MW molecule with t ½ of 2 hrs • Normal concentration of factor VII: 10-20 nm • 1% of factor VII circulates in activated form • Standard dose in hemophilia 90 ug/kg – maximum concentration 50 nm – 500 x normal concentration of VIIa • Dosing q 2-3 h to maintain hemostatic levels Expanded Use of VIIa • • • • • • • • • • • Factor VII deficiency Other factor deficiencies (FXI, acquired FX) Von Willebrand’s disease Thrombocytopenia Platelet function defects Reversal of oral anticoagulation Coagulopathy of liver disease Surgical bleeding Surgical prophylaxis Intracranial hemorrhage Trauma VIIa in Partial Hepatectomy: Placebo Controlled RCT Design: • RCT comparing 20, 80 ug/kg or placebo in noncirrhotic patients with 2nd dose for OR > 6 hrs • Monitored for bleeding for 7 days Results: • 185 patients Lodge et al. Hepatology 2002;36:Abst177 VIIa in Partial Hepatectomy Placebo Blood loss 1000 % transfused 37% Thrombosis 3 20 ug/kg 800 41% 3 Trend but no significant difference 80ug/kg 700 25% 3 Effect of rVIIa on Perioperative Blood Loss During Prostatectomy Friederich. Lancet 2003 Design • 36 pts randomized to placebo or rVIIa (20 ug/kg or 40 ug/kg) • Followed for 10 days post-op Results Placebo 20 40 Blood loss (ml) RBC units % transfused OR time (min) ug/kg ug/kg 2688 1235* 1089* 1.5 58% 180 0.6* 38% 126 0* 0%* 120 RecombinantVIIa for Adjunctive Hemorrhage Control in Trauma Patients • 7 patients with coagulopathic bleeding treated under compassionate use program • Age 17-75 • No atherosclerosis/thromboembolic disease • Received 20-70 units RBCs + FFP and platelets Intervention • 1-3 doses of 60-120 ug/kg of rVIIa Matinowitz et al. J Trauma 2001;51:431 Recombinant VIIa for Adjunctive Hemorrhage Control in Trauma Results • Bleeding stopped/reduced in all patients • Further transfusions limited to 1-2 units of RBCs • Significant shortening of coagulation tests • 4 of 7 patients survived • No thrombotic events Multicentre RCT of rVIIa in Trauma • 280 pts with blunt or penetrating trauma • All patients receive 3 doses – 200 ug/kg followed by 100ug/kg 1h and 3h later trauma Arrive at ER randomize 0 6 rVIIa 8 48h 30d 48h 30d placebo Units of RBCs Transfusion ICU/Hosp LOS Survival Adverse Events Boffard et al. J Trauma. 2005: 59: 8-15 Multicentre RCT of rVIIa in Trauma • Non significant reduction in RBC units transfused – Significant reduction in RBC units (2.6 units) and incidence of massive transfusion (14% vs 33%) in blunt trauma when early deaths excluded • Similar overall survival - 25% vs 30% – Composite outcome incl organ dysfunction showed increased trend favouring rVIIa (29 vs. 43%) • No difference in adverse events Boffard et al. J Trauma. 2005: 59: 8-15 Complications Associated with Factor VIIa FDA report of adverse events from trials and voluntary reporting from 1999-2005 • 220 thromboembolic complications – 193 in non-hemophiliac patients • 129 arterial thrombosis • 103 venous thrombosis • Approx. 50% of reports within 24 hrs of infusion • 43 of 67 deaths reported secondary to thrombosis