* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Book 5
Entropy of mixing wikipedia , lookup
Internal energy wikipedia , lookup
Temperature wikipedia , lookup
Heat transfer physics wikipedia , lookup
Thermodynamics wikipedia , lookup
Thermodynamic system wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
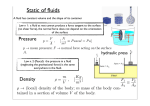
PHYSICS 125 Lecture Notes Book 5 Prepared by Kai Wong Table of Contents 22. Fluid Pressure …………………………………………………………………. 2 23. Buoyancy ……………………………………………………………………….. 9 24. Temperature and Heat ………………………………………………………. 14 25. Kinetic Theory of Gases ……………………………………………………… 21 26. First Law of Thermodynamics ……………………………………………….. 25 27. Second Law of Thermodynamics ……………………………………………… 34 1 22. Fluid Pressure Density The density of a substance is defined by m mass V volume It has the SI unit of kg/m3. Sample values of densities: water 10 3 kg / m 3 1 g / cm 3 steel 7.8 10 3 kg / m 3 air 1.29 kg / m 3 Example: What is the radius of a sphere made of steel that has a mass of 100g? m 0.1 Solution: V 1.28 105 m3 7800 From V 4 3 r 3 r 3 3V 1.45 10 2 m 1.45cm 4 Pressure When a force F acts perpendicularly on an area A , the pressure it exerts on the surface is defined as F A P F force A area The SI-unit of pressure is N/m2, which is written as Pa (pascal). Two other units are 1 ATM atmosphere 1.013 10 5 Pa 10 5 Pa 4.445 N 1 psi 1 lb / in 2 6890 Pa 2 1 m 12 3.281 1ATM 14.7 psi 2 Example: A woman weighing 700N stands on the heel of one high-heel shoe. The area of the heel is 1cm2. How large is the pressure the heel exerts on the floor? 2 Solution: A 1cm 2 10 2 m 10 4 m 2 P F 700 7 10 6 4 7 10 6 Pa ATM 70 ATM A 10 10 5 Fluid Pressure A fluid (gas or liquid) in a container exerts forces on the wall of the container. It also exerts forces on the surface of a submerged object. In fact, even if the submerged object is replaced by the fluid itself, the surface of the latter fluid still experiences the same forces. To define the pressure of a fluid at any point inside the fluid, we construct mentally a small planar surface (say in the shape of a coin) of area A around that point. The fluid on one side of the surface will push on the fluid on the other side with a force F, in a direction perpendicular to the surface. The pressure is then calculated from P F A . It turns out as a fundamental property of a fluid that P is independent of the orientation of the surface chosen. F A Pascal Principle For an enclosed fluid not under the influence of gravity, the pressure is the same everywhere in the fluid if it remains at rest, because a pressure difference would set up motion. Therefore, if a change of pressure P occurs at one location, the same change occurs everywhere. This is called Pascal principle. Consider a fluid enclosed in a vessel with two pistons as shown. A piston is capable of moving without leaking the fluid. If the forces on the pistons due to the fluid are denoted 3 by F1 and F2 , while the areas of the pistons are A1 and A2 , and if P is the pressure of the fluid everywhere, we have P F1 A1 P F2 A2 F1 F2 A1 A2 A2 A1 F1 F2 To push the left piston in, we need to apply a force equal to (actually slightly more than ) F1 . If the fluid is incompressible, its volume cannot change. The right piston is therefore forced out with a force F2 . If A1 is less than A2 , then F2 is larger than F1 . In this way, a small force is transmitted through the fluid and amplified to a lager force. This phenomenon is exploited in a hydraulic lift: F A2 A1 W F2 F1 A F W W 2 F F if A2 A1 A1 A2 A1 Variation of pressure with height Consider a fluid under the influence of gravity. First we show that the pressures at the same level are equal. Label the points at the same level by 1 and 2, and construct identical vertically oriented small areas around the points. 4 A A F1 F2 1 2 Consider the fluid inside a horizontal cylinder formed between the surfaces. Since this fluid is in equilibrium, the sum of forces on it is zero. Balancing the push forces on either sides of the two surfaces lead to F1 F2 Or P1 A P2 A P1 P2 Next consider two points so that point 1 is above point 2 by a height h. Construct identical small horizontal surfaces at these points, and consider the condition of equilibrium for the fluid inside a vertical cylinder between these surfaces. The upward push on the bottom surface is now balancing the downward push on the top surface plus the weight of the fluid column. F1=P1A h W F2=P2A F2 F1 W P2 A P1 A mg Since the volume of the column is V hA , we can write 5 m V hA where is the density of the fluid. Therefore P2 A P1 A hAg which simplifies to P2 P1 gh The pressure at 2 is higher than at the point 1 in order to support the weight of the fluid above. Combining the last result with the earlier one regarding pressures at the same elevation, we see that the formula P2 P1 gh applies even if the point 2 is not directly below the point 1. Example: What is the change in pressure in going down 10m of water in the ocean? Solution: Choose the point 1 at the surface and point 2 at 10m below. h 10m 10 3 kg / m 3 g 10m / s 2 P2 P1 gh 10 5 Pa 1 ATM Example: What is the pressure 20m deep in the ocean? Solution: P2 P1 gh 10 3 10 20 2 10 5 Pa 2 ATM P2 P1 2 3 ATM Toricelli’s experiment Fill a long glass tube with mercury and invert it in a trough of mercury. The mercury in the tube will fall until its height is h . The value of h can be calculated from the density of mercury and the pressure of the atmosphere. 1 h 2 6 Choose the point 1 to be at the top of the mercury column. The pressure P1 0 because the space above it is vacuum. Choose the point 2 to be at the bottom of the column. The pressure where PA is atmospheric pressure, because pressure is the same at the P2 PA same level and the mercury in the trough is exposed to atmosphere. Applying the formula for pressure variation of height, we find PA gh where 13.6 10 3 kg / m 3 is the density of mercury. The height of the column is therefore PA 1.013 10 5 h 0.76m 76cm g 13.6 10 3 9.8 If water is used instead of mercury, h PA 1.013 10 5 10m g 10 3 9.8 Equalization of water level In a connected body of water exposed to the atmosphere, the water surface is always at the same height. For example, in the odd-shaped vessel shown, the points A,B and C in the fluid are chosen at the same height, and therefore have the same pressure: PA PB PC The points A’, B’, and C’ at the water surface also have the same pressure, namely, the atmospheric pressure: PA PB PC Patmosphere Therefore the height h of the surface is the same, being given by h PA PA PB PB PC PC g g g 7 B’ C’ A’ h A C B Pressure gauge A U-tube filled with mercury with one end exposed to the atmosphere can be used to measure the pressure of a gas in a container connected to the other end of the U-tube as shown. PA P h If P is the pressure of the gas, PA the atmospheric pressure, and h is the height of one surface of the mercury column above the other, applying the formula for pressure variation of height gives P PA gh The height h can therefore be used to measure the pressure difference P PA , the amount of pressure above atmospheric, which is known as the gauge pressure. We thus have another unit of pressure 1 mm mercury gh 13.6 10 3 9.8 10 3 133 Pa 1 ATM 760mm mercury When the tire pressure is quoted as 32 psi, we should add the atmospheric pressure of 14.5psi to yield 45.5psi.for the absolute pressure. 8 23. Buoyancy Buoyancy Force An object fully or partially submerged in a fluid under gravity experiences an upward force due to the fluid. This force arises from the fact that the pressure of the fluid is higher at the bottom of the object than at the top. F1 A 1 f h 2 F2 Consider a solid in the form of a rod of length h and cross-sectional area A, completely submerged in a fluid of density f . All faces of the solid are subject to compressive pressure forces. The net upward component of theses forces is B F2 F1 P2 A P1 A P2 P1 A f ghA f gV m f g W f Therefore, Buoyancy force = weight of fluid displaced by the submerged volume of solid This is known as Archimedes’ principle. It applies to objects of arbitrary shapes, as well as to objects only partially submerged. Apparent weight Consider an object completely submerged in a liquid and resting on the bottom of the container. The forces on the object consist of its weight downward and two upward forces: buoyancy and normal force. 9 B FN W At equilibrium, B FN W 0 FN W B The normal force is the apparent weight, and is reduced from its true weight by an amount equal to the buoyancy force. B T W Similarly, if the object is suspended by a string, the tension T in the string is less than the weight, and is also a measure of apparent weight: T W B The consideration from normal force applies also to partial submersion, as the following example shows: Example: A 20-kg piece of concrete is 80% submerged in water. What is the apparent weight? (density of concrete = 2200 kg/m3) 10 Solution: First find buoyancy force B from Archimedes principle. For this we need the volume submerged. Volume of concrete block V 3 m concrete 20 9.1 10 3 m 3 2200 Vsubmerged 0.8V 7.3 10 m From Archimedes principle, B water gVsubmerged 1000 9.8 7.3 10 3 72N W mg 20 9.8 196 N FN W B 196 72 124 N (apparent weight) 3 Determination of density from apparent weight The weight of a solid with volume V and density solid is given by W solidVg The buoyancy force on the same solid when it is completely submerged in water is given accordingly to Archimedes principle by B waterVg By definition, the specific gravity S.G. of a substance = density of the substance /density of water It follows from the above two relations that S .G. of solid solid solidVg W water waterVg B Thus we can determine the specific gravity of the solid if we can measure the buoyancy force. The latter can be determined from the apparent weight W a of the solid when it is completely immersed in water using the equation B W Wa A balance scale can be used to measure the apparent weight of the solid while under water in an arrangement as shown: 11 B W Wa : Example: The emperor’s crown weighed 2 lb in air and 1.8 lb under water. Is it made of gold? Solution: Weight of crown W 2 lb Apparent weight in water Wa 1.8 lb Buoyancy force B W Wa 2.0 1.8 0.2 lb W 2.0 S .G. of crown material 10 B 0.2 Density of crown material solid S .G. water 10 10 3 kg / m 3 10 4 kg / m 3 Since the density of pure gold is golkd 19,300kg / m3 , the emperor had been cheated. Floatation The criterion whether a solid will float or sink in a liquid is given by Sink : solid liquid Float : solid liquid This can be derived as follows. Consider the solid to be completely immersed in the liquid and not in contact with the bottom of the container. B V W Net upward force B W liquidVsolid g solidVsolid g liquid solid Vsolid g 12 The object will start to rise (float) if the net force is upward, which happens if liquid solid . Otherwise, it moves downward, or sink. In the former case, the object will rise until part of its volume protrudes into air, so that it is only partially submerged. The buoyancy force is therefore reduced, and can balance the weight, as the following example shows: Example: What fraction of the volume of an iceberg is beneath water? ( ( ice 917kg / m 3 ) Solution: B W At equilibrium, B W Vsubmerged Or, waterVsubmerged g ICE Vice g ice 917 0.917 water 1000 Vice Tip of iceberg 8.3% of total volume. Example: A raft floating on water has a bpttom that measures 2m 1m .w much does it sink in level when a 30kg child steps on it? Solution: Without child, B Wraft With child, B Wraft Wchild Additional buoyancy force B B B Wchild From Archimedes principle, B water Vsubmerged g Vsubmerged Wchild m 30 child 0.03m 3 water g water 1000 Since Vsubmerged Ah h Vsubmerged A A area ofbottom, b change in water level 0.03 0.015m 1.5cm 2 1 13 24. Temperature and Heat Temperature Scales Most substances expand when heated, and contract when cooled. The amount of expansion or contraction can be used to measure temperature. In a mercury thermometer, mercury is trapped in a glass container consisting of a bulb connected to a thin tube. When the bulb is immersed in a mixture of ice and water, the level of mercury in the tube is marked off and labeled 0°C. When it is immersed in a mixture of steam and water, the corresponding mark is labeled 100°C. The reason for choosing such mixtures to define 0°C and 100°C is because the temperatures of these mixtures are fairly constant, not being influenced much by the heat absorbed from or released to the environment. The length between these marks is then divided into 100 equal parts. When the bulb is in contact with the object whose temperature is being measured, the temperature is indicated by the level of mercury. In this way, the centigrade scale, or Celsius scale, is defined. It is a property of mercury (as well as many other liquids) that the same reading is obtained for thermometers thus constructed. 100 ºC 212 ºF steam+water TC ºC TFºF 0 ºC 32 ºF ice+water In the Fahrenheit scale, the marks for ice-water and steam-water mixtures are labeled 32°F and 212°F respectively, and the length between the marks is divided into 180 equal parts. The notations for differences of temperatures in the two scales are C° and F°. We have 1C 1`80 9 F F 100 5 If TC and TF denote the temperatures in the Celsius and the Fahrenheit scales, we have, from the construction of the scales, TC 0 TF 32 100 180 14 9 This can be solved to yield either TF 32 TC 5 5 Or TC TF 32 9 The constant volume gas thermometer relies on the increase of gas pressure with temperature for temperature measurement. It consists of a gas trapped inside a glass bulb connected to a U-tube filled with mercury. The U-tube is open to the atmosphere on the other end, and is partly made from rubber tubing so that it can be raised or lower to ensure that the volume of the trapped gas remains constant as the bulb is introduced into various temperature environments. The height of the mercury column is a measure of the gauge pressure of the gas. PA P P=PA+ρgh h Experiments show that (1) A plot of P versus TC (temperature in Celsius) is a straight line. (2) Extrapolating the straight line to P=0 results in the same temperature of -273 ºC independently of (a) the amount of the trapped gas (b) the nature of the trapped gas P -273ºC TC TC+273 TC (ºC) If we define temperature in Kelvin scale TK (K ) by TK TC 273 15 (Note that we do not use the degree notation with K) , then the plots of P versus TK are straight lines passing through the origin: P 0K TK(K) As a result, P is proportional to TK : P TK , with the proportionality constant depending on the nature and the amount of the trapped gas. The Kelvin scale not only simplifies formulas for the properties of gases, it turns out also to have an absolute meaning from thermodynamics. For this reason, it is also called the absolute temperature, No object can have negative temperature in the Kelvin scale, Heat Capacity To raise the temperature of an object, we can bring it in contact with another with higher temperature. For example, water in a pot is heated through contact with the hot gas from a gas stove. The influx of heat causes the temperature to go up, while an outflux of heat causes it to go down. In the past, people thought that heat is a substance, flowing from high temperature to low temperature like water flowing from high to low elevations. The increase of temperature of an object is proportional to the quantity of heat absorbed by the object, while the decrease of temperature is proportional to the quantity of heat released. This relation is expressed by the equation Q CT T T f Ti where Q is positive if heat is absorbed and negative if heat is released. The constant of proportionality C is called the heat capacity of the object. Objects with higher heat capacity require more heat to have their temperature raised by the same amount. Conversely, for the same amount of heat absorbed, such objects will experience less increase in temperature. 16 ΔT Q For objects made of pure substances, the heat capacity is expected to be proportional to the mass. If the mass of the object is m , the relation between heat absorbed and temperature rise is written as Q mcT where the quantity c (lower case) is called the specific heat capacity, or just specific heat, of the pure substance. In the caloric theory, heat is measured in a unit called the calorie. This is defined to be the quantity of heat required to raise the temperature of one gram of water by 1 C . Substituting into the relation between Q and T : 1cal 1g c 1C Therefore, the specific heat of water is c water 1 cal / g C Calorimetry The caloric theory also postulates that heat is indestructible so that, for instance, the heat released by an object must be equal to the heat absorbed by all others with which it comes into contact. This can be expressed by the equation Q 0 where the summation is over all objects in thermal contact with each other, and Q is the heat absorbed by each, with a negative Q meaning heat released. In terms of specific heat and temperature change for each object, assumed to be made of pure substance, mcT 0 This can form the basis of determining specific heat as the following example shows: 17 Example: A 50g sample of aluminum is heated to 100°C in a pot of boiling water. It is then introduced into 20g of water initially at 25°C in a well insulated container. The final temperature is 51°C. Determine the specific heat of aluminum. Solution: The equation mcT 0 becomes 50c Al 51 100 20cwater 51 25 0 Using c water 1cal / g C , this becomes 2450c Al 520 0 so that c Al 520 0.21cal / g C 2450 Such experimental procedures that account for heat flows using apparatus that minimize heat leak to the environment form the basis of calorimetry. The Mechanical Equivalent of Heat The temperature of an object can also be raised by rubbing. The force doing the rubbing does work against friction, and its effect on the object is equivalent to the absorption of heat. It is found that a given amount of work is always converted to the same amount of heat. Joule’s experiment demonstrates this and establishes how many Joules of work is equivalent to 1 calorie. The apparatus used by Joule is as shown, consisting of paddles that can be set to rotate by a falling weight, thus churning the water in the thermally insulated container. The equivalent heat in calorie absorbed by the water can be calculated from a reading of the rise in temperature and the mass of the water. The corresponding amount of work measured in Joule is obtained from the height through which the hanging weight falls. In this way, it was found that 1 calorie is equivalent to 4.186 J 18 which is called the mechanical equivalent of heat. Since work and energy have the same unit, we can regard heat as just a flow of energy, or energy in transit. Objects heat up when they absorb energy. Converting from calorie to J and gram to kilogram, the specific heat of water can be expressed as c water 1cal / g C 4.186 J / 10 3 kg C 4186 J / kg C Example: What is the final temperature when a 75g silver ring at 100°C is dropped into a cup containing 10cc water at 20°C? (The specific heat of silver is 235J / kg C ) Solution: The mass of 1cc of water is 1g. Let T f be the final temperature of the water and ring, the equation mcT 0 becomes 0.075 235 T f 100 0.010 4186 T f 20 0 17.6T f 1783 41.86T f 837 0 Tf 1783 837 43.7 C 17.6 41.9 Latent Heat All substances can exist in the three phases of solid, liquid, and vapor, depending on the temperature (and also the external pressure). The transformation from solid to liquid is known as melting, and the reverse freezing. The transformation from liquid to vapor is known as vaporization, and the reverse condensation. For a given pressure, there is a unique temperature at which the solid and the liquid phase can coexist. This temperature is known as the melting point. Similarly, the temperature at which liquid and gas can coexist is known as the boiling point. During phase transition, the temperature remains at either the melting or the boiling point, although heat is absorbed or released. Heat is absorbed by a substance undergoing melting and vaporization, and is released to the environment during freezing and condensation. The amount of heat absorbed or released is proportional to the mass of the substance. Latent heat of vaporization is the amount of heat absorbed by unit mass (1 kg in SI unit) of a substance being transformed from the liquid to the vapor phase. The same amount of heat is released when the substance condenses. Latent heat of fusion is the heat absorbed by unit mass of the substance being transformed from solid to liquid phase, and is released during freezing. These are known as latent heats because there is no change of temperature involved. The following values apply to water: Latent heat of fusion LF 80 cal / g 3.35 10 5 J / kg Latent heat of vaporization Lv 538 cal / g C 2.26 10 6 J / kg C 19 The rather large value of the latter is responsible for the phenomenon of scorching. The skin in contact with steam absorbs a huge amount of energy from the condensation of steam into water. If m is the mass of a substance that has undergone phase change, and L is the corresponding latent heat, the amount of heat absorbed or released is given by Q mL The following plot of temperature against heat absorbed pertains to 1 g of the water substance starting as 10°C subfreeze ice. T(°C) 100°C water+steam steam water 0°C 80cal 536cal Q(cal) Ice+water -10°C ice Example: How much ice is melted when 20 kg of ice 0°C absorbs 106 J of heat? Solution: Amount of heat to melt all 20 kg of ice is Q mL fusion 20 3.35 105 6.7 106 J which is more than 106 J. Therefore, the ice is not completely melted. 10 6 3 kg The amount melted = 3.35 10 5 Example: If in the previous example, the amount of heat absorbed is 107 J, what is the final temperature? Solution: Since 107J is more than 6.7 10 6 J , the ice has completely melted. The heat anbsorbed goes to both melting the ice and heating up the resulting water. Fron Q mL mcT Q mL 10 7 6.7 10 6 T 39C mc 20 4186 Therefore the final temperature is 39°C. 20 25. Kinetic Theory of Gases Derivation of Ideal Gas Law The ideal gas law can be written in another form if we replace the mole number n by the relation n N NA where N is the number of molecules and N A is Avogadro’s number. The result is PV NkT where k R 1.38 10 23 J / K NA can be regarded as the ideal gas constant per molecule, and is known as the Boltzmann constant. The theory of gases has been developed as a phenomenological theory in which concepts and relationships are extracted from experiments. For example, temperature is such a concept. A more fundamental theory known as the kinetic theory is able to explain the ideal gas law in terms of purely mechanical concepts such as forces and Newton’s laws of motion. In this theory, the gas is made up of a collection of molecules which are constantly moving inside the container. The number of molecules is very large. When a molecule strikes the wall of the container, it bounces back just like a tennis ball bouncing back from the ground it hits. In so doing, the molecule delivers a force on the wall, and this the origin of pressure of the gas. To obtain a quantitative relation for the pressure, we consider for simplicity a cubical volume of side L containing N molecules of the same mass m . The volume is V L3 . We assume the molecules are equally divided among six populations, traveling respectively with the same speed v in the x directions, y directions, and z directions. We focus our attention on an area A on one face of the cube, and ask how many molecules will hit the face in a time interval t . These would be molecules moving toward that face and within the distance vt from the face. Such molecules are shown in red in the figure. (Molecules further away from the face shown in black could not reach the face in the interval t ) They are all in a volume vtA . 21 vt A Since the number density of molecules is N V , the number of molecules in this volume and traveling toward the face is therefore 1N vtA (=number of molecules striking area A in time t ) 6V Assuming elastic collision with the wall, each molecule bounces back with the same speed but in the opposite direction, and so momentum loss in each collision = 2mv The loss of momentum of the colliding molecules is p 1N 1N vtA2mv mv2 At 6V 3V Force on the colliding molecules is p 1 N mv2 A t 3 V which, by Newton’s third law, is also equal to the force on the face of the cube. The pressure on the face is therefore P F 1N 2N mv2 K A 3V 3V where K mv2 2 is the kinetic energy of the molecule. We thus have PV 2 NK 3 If we compare this relation with the ideal gas law, we see that they are the same if we make the identification 22 K 3 kT 2 Thus, the temperature of an ideal gas is related to the kinetic energy of the individual molecules, and we have found a mechanical explanation of the ideal gas law. In a more complete version of kinetic energy, the molecules do not all move with the same speed, and certainly do not move in only six directions. But the average kinetic energy K is still given by K 1 2 3 mv kT 2 2 From this we find the root mean squared (rms) velocity vrms v 2 3kT m as the typical speed of a molecule. Thus the rms velocity increases with temperature, and is smaller for heavier molecules. Example: Find the rms velocity of oxygen molecules at 20ºC. Solution: The molecular weight of oxygen gas O2 is 2 16 32 m 32 1.66 1027 kg vrms 3kT m T 273 20 293K 3 1.38 1023 293 478m / s 32 1.66 10 27 Under ordinary circumstances, gas molecules traveling at such high speed are continuously bumping into each other and change course. They follow zigzag paths with tiny straight sections called the mean free path, which is the path length between successive collisions. The time between collisions is very short. Internal Energy Multiplying the average kinetic energy of a molecule by the total number of molecules, we obtain the total kinetic energy of the gas: U NK 3 3 NkT nRT (monatomic gases) 2 2 This is the internal energy of a monatomic gas, such as helium, neon, etc., whose molecule consists of a single atom. For diatomic gases, such as oxygen O2 , nitrogen 23 N2 , the molecule is like a short bar, and can tumble as it travels. There is additional kinetic energy associated with the tumbling motion, which is describable as rotation. For such gases, the internal energy is given by U 5 nRT 2 (diatomic gases) In general, the internal energy of a macroscopic body is the sum total of the kinetic energy and the potential energy of the interaction between the molecules. For an ideal gas, the molecules are free from interaction between each other. There is therefore no contribution from the potential energy of interaction to the internal energy. As the temperature is lowered, the speed of the molecules can no longer overwhelm the influence of the force of attraction between the molecules. The gas will no longer behave as an ideal gas, and its internal energy will have significant contribution from the intermolecular potential energy. Thus, while a mass of steam at high temperature can be considered as an ideal gas, and has the aforementioned expression for internal energy, when it condenses to water, that expression can no longer apply. The internal energy of the water has a large influence from the potential associated with the cohesive force between its molecules. Using the ideal gas equation PV nRT , an alternative expression for the internal energy is 3 2 PV U 5 PV 2 monatomic diatomic Example: Find the internal energy of 1L of oxygen at STP. Solution: Method 1: Since at STP, the volume occupied by one mole of an ideal gas is 22.4L, the number of moles in 1L volume is n 1 0.045 22.4 Also, T 273K . 5 5 U nRT 0.045 8.31 273 255 J 2 2 Method 2: P 1ATM 1.01 105 Pa V 1L 103 m3 U 5 PV 253 J 2 24 26. First Law of Thermodynamics A thermodynamic system is composed of one or more macroscopic objects, and as such is very general. It can be a gas in a container, a glass of water, the chair you are sitting on, or even a human being. The only requirement is that a large number of molecules are present in the system, which is what we mean by macroscopic. When two systems of different temperatures are brought into contact with each other, heat will flow from one to the other, and will cease only when the two reach the same temperature. It will take some time before this happens. In this case, we say that the two systems are in thermal equilibrium. Thus, the temperature of a system indicates whether or not the system can be in thermal equilibrium with another system. The zeroth law of thermodynamics states that two systems are thermal equilibrium when they have the same temperature. We also introduce the concept of a system itself in thermodynamic equilibrium. This means that the various parts of the system are in mechanical as well as thermal equilibrium. In particular, the temperature of a system in thermodynamic equilibrium is the same everywhere in the system. In this regard, the human being is not in thermodynamic equilibrium, because different parts of the body have different temperature. If a system is isolated so that it does not exchange heat and does not interact with its environment, it would reach thermodynamics equilibrium after being disturbed. The time for this to happen is called the relaxation time. The state of a system in thermodynamic equilibrium is characterized by a number of variables called the state variables. For a gas, its pressure, volume, and temperature are examples of state variables. Experiments showed that a system in thermodynamic equilibrium has a state variable called its internal energy. The internal energy is composed of the microscopic kinetic energy of the molecules and the potential energy of interaction among the molecules. For a system consisting of a fixed number of molecules, the internal energy can be changed in two ways: (1) flow of heat into and out of the system (2) work done on or by the system Clearly, the internal energy increases if it absorbs heat, and decreases if it releases heat. When the system does work to the environment, such as happens when the hot gas inside a cylinder in an internal combustion engine expands and pushes the piston, or when a person pushes on the paddles while riding an exercise bike, the internal energy decreases. In this case, we say that work is done by the system. Work can also be done on the system. If the system is a glass of water, work is done on it if the water is stirred. Work is also done on the gas inside a container if the container is squeezed so that its volume is reduced. Let 25 U = internal energy of the system Q = heat absorbed by the system W = work done by the system with the understanding that a negative value of Q means heat is released, and a negative value of W means that work is done on the system, the change of internal energy is given by U Q W This is known as the first law of thermodynamics, and can be pictorially represented: ΔU W Q It is interesting to compare the first law of thermodynamics with the work energy theorem in mechanics. The latter states that the total work done by the all the forces acting on an object is equal to the change of kinetic energy, and is written as: K W The total work done by the forces can be regarded as the work done on the object. The work done by the object is therefore the negative of W . Thus K work done by the object and is similar to the first law of thermodynamics except there is no flow of heat in mechanics. Example: A sample of two moles of an ideal monatomic gas undergoes a change during which it receives 240J of heat and 60J of work is done on it. Find the change in temperature. Solution: Heat received by the system Q 240 J Work done by the system W 60 J Change of internal energy U Q W 240 60 300 J 26 3 nRT for monatomic gases, 2 3 2 U 2 300 U nRT T 12 K 2 3 nR 3 2 8.31 Using U P-V Diagram of An Ideal Gas The state variables P, V , T and n of a gas in thermodynamic equilibrium are not independent. For an ideal gas, they are related by the ideal gas law. Let’s write the ideal gas law in the form P nRT V and plot P against V at a fixed temperature. We obtain a curve that is a hyperbola:: P V If we increase the temperature and make another plot, we obtain another hyperbola lying above the original one. If we decrease the temperature, we obtain a hyperbola that lies below. In this way, we construct a family of hyperbolas. On each hyperbola, the temperature is the same, but pressure and volume can change. Each hyperbola is called an isotherm. In the P-V diagram for a fixed mass of gas, each point represents a state in thermodynamic equilibrium. A thermodynamic process is a course of events involving change of states. If the process is slow enough so that the system has time to relax to thermodynamic equilibrium as the change progresses, the process is called quasi-static. Such process can be represented by a path in the P-V diagram, as illustrated by the red curve in the following diagram, which also shows a family of isotherms. 27 P 2 1 V Work done by a gas Consider a gas inside a cylinder with cross-section area A fitted with a piston. Δx P A Let P be the pressure of the gas. Then the force acting on the piston due to the gas is F PA Suppose the piston moves through an infinitesimally small distance x . During this displacement of the piston, the volume change of the gas is small, and its pressure is essentially unchanged. The work done by the gas is therefore W Fx PAx PV where we have used the fact that V Ax is the change in volume of the gas. It turns out that the above formula for the work done by a gas during an infinitesimal change of volume is applicable to any shape of the container. The work by the gas is positive when the gas expands, and negative when the gas contracts. When the volume change is not small, the pressure of the gas changes in the course of the volume change, and the above formula no longer applies. In this case, the work depends on the particular process during which the volume changes. It can be computed by dividing the overall volume change into many small parts, for which the incremental work can be computed by the above formula, and summing the contributions from these infinitesimal works: W PV In the P-V diagram, the product PV is equal to the area of the rectangle as shown. In the limit when V is very small, the total work is therefore equal to the area underneath the P-V curve for the process from the initial volume V1 to the final volume V 2 . It should 28 be noted that if V1 is less than V 2 , the work done by the gas is the negative of the area underneath the P-V curve. P W=A A V1 P V2 W A V1 ΔV V2 V A V2 22 V1 Thermodyanamic Processes of ideal gas (1) Isothermal Process During an isothermal expansion of an ideal gas, the temperature is kept constant at T, while the volume expands from V1 to V 2 . The path of the process in the P-V diagram is part of the isotherm at the temperature T. The work done by the gas, which is equal to the area underneath the P-V curve, can be obtained by calculus. The result is W nRT ln V2 V1 V1 V2 The same formula applies to isothermal contraction, for which V1 is larger than V 2 , and the work by the gas turns out to be negative. Since the internal energy of an ideal gas depends only on n and T , it does not change during an isothermal process: U 0 29 Using the first law of thermodynamics, U Q W , the heat absorbed by the gas is Q W In this case, we can say that the expanding gas draws on the heat it absorbs from its environment to do work. Example: How much heat is released when 3 moles of an ideal gas is compressed isothermally at the temperature 50ºC so that its volume is reduced by a factor of ten? Solution: n 3 T 273 50 323K V2 V1 1 10 0.1 W 3 8.31 323 n0.1J 3 8.31 323 2.30 1.85 10 4 J Since U 0 , the heat absorbed is Q W . Therefoe heat released is 1.85×104 J. (2) Isobaric Process In an isobaric expansion, an ideal gas changes its volume from V1 to V 2 while maintaining the pressure at P. The P-V diagram is a horizontal straight line, so that the work done is W PV2 V1 PV if we define V V2 V1 . P V V1 V2 The temperature changes because the path on the P-V diagram brings the state from one isotherm to another. Writing the ideal gas law in the form T PV nR the temperatrure change is P V T nR If the gas is monatomic, its internal energy will change by the amount U 3 nRT 2 and it will absorb an amount of heat according to the first law of thermodynamics by 30 Q U PV 3 5 nRT nRT nRT 2 2 Therefore, the molar heat capacity at constant pressure is, by definition, cp Q 5 R nT 2 J / mol K Replacing 3 2 in the formula for U by 5 2 , the same derivation shows that for diatomic gases, cp 7 R 2 J / mol K Example: Find the change in internal energy when 1 kg of water at 100ºC is converted into steam at the same temperature under a pressure of 1 atmosphere, assuming that the steam can be considered an ideal gas. (The heat of vaporisation of water is 2.26×106J) Solution: The process is isobaric. The initial volume is essentially zero considering the much larger volume of the steam generated. The work done by the system is therefore W PV nRT where we have used the ideal gas law in the last equality. Since the molecular weight of water (H2O) is 18, the number of moles is n 1000 55.6 18 and the temperature is 373K. W 55.6 8.31 373 1.7 10 5 J The change of internal energy is U Q W 2.26 10 6 1.7 10 5 2.110 6 J almost all the heat absorbed goes to increase the internal energy (3) Isochoric Process 31 In an isochoric process, the temperature of a ideal gas changes from T1 to T2 with the volume held constant at V . The process is represented by a vertical line segment in the P-V diagram. P V V In this case, the work done by the gas is W 0 so that from the first law, the heat absorbed is equal to the change of internal energy Q U The latter can be computed from U 3 nRT 2 for monatomic gas. Hence the molar heat capacity at constant volume is cV Q 3 R nT 2 for a monatomic gas. For a diatomic ideal gas, the result is cV Q 5 R nT 2 The molar heat capacity at constant pressure is higher than at constant volume, because in the former case, the heat absorbed is used up to do work against the environment in addition to increasing the internal energy. Example: Find the specific heat capacity of oxygen at constant pressure and constant volume. Solution: Since the molecular weight of oxygen (O2) is 32, the number of of moles in 1 kg of oxygen is n 1000 32 31.25 . The two specific heat capacities are 5 cV 31.25 8.31 649 J / kg C 2 7 c P 31.25 8.31 909 J / kg C 2 32 (4) Adiabatic Process This is a process in which expansion or compression takes place without heat exchamge with the environment. It can be arranged if the gas is contained in a thermally insulated vessel whose volume can change. It also occurs in an unsilated environment if the compression or expansion is fast enough so that there is no time for heat exchange to take place to a significant degree. For an idiabatic process, Q0 so that U W In an adiabatic expansion, the gas does work against the environment, so that W is positive. It therefore loses internal energy and cools down. Conversely, in an adiabatic compression, work is done on the gas by the environment, so that W is negative, resulting in an increase of internal energy and hence temperature. The Santa Ana conidtion results from rapid rise of pressure and the consequent adiabatic conmpression in the atmosphere. If the volume expands from V1 to V 2 adiabatically, both pressure and temperature will change. The final state will lie on an isotherm lower than that of the initial state. The path of an adiabatic process is steeper than an isotherm as shwon in the figure. It can been shown that in the pressure and volume are related in this case by P1V 1 P2V2 P V where is the ratio of specific heat at constant pressure and constant volume: c P 5 3 cV 7 5 monatomic diatomic 33 27. The Second Law of Thermodynamics Irreversible Transformations Many changes or processes in mechanics are reversible. If two billiard balls collide and fly apart, it is possible to reverse the paths and velocities of the balls after the collision and recover their paths and velocities before the collisions. A planet may go around the sun in one direction in its orbit. But the motion in which it traverses the same orbit but in the opposite direction is perfectly acceptable. Watching a movie made of such processes, you cannot tell whether the movie is being run forward or backward. However, for changes involving macroscopic objects, many are irreversible. A transformation in a thermodynamic system is called irreversible if the original state cannot be recovered in such a way that no other changes occur anywhere else. The following are three examples: 1. The heating of an object by friction. To make this precise, consider Joule’s experiment in which water is heated up by a falling weight driving paddles to stir up the water. It is impossible to make the water cool back to the original temperature and raise the weight back to its original height, with nothing else happening in the rest of the environment. Since in the Joule experiment, the work done by gravity on the weight is completely transformed into heat, the reverse change of transformation of heat completely into work is impossible. 2. The transfer of heat from a hot object to a cold object. In this case, it is not just impossible for the heat to flow directly back through thermal contact, which is obvious because by definition heat flows from high to low temperatures. In fact, no means can be found to take the heat from the cold object and deliver it to the hot one, with no other change in the environment. 3. The expansion of an ideal gas in a fixed volume container that allows no heat flow in and out. Imagine the container to be partitioned at the beginning, with the gas occupying one side and vacuum the other side. On opening the partition, the gas expands to occupy the full volume of the container. On the other hand, once filling up the container, the gas never by itself shrinks back to its original volume, leaving vacuum behind. We note that the temperature of the gas has not changed when it expands to fill the vacuum, because it does no work in expanding into a vacuum, and there is no exchange of heat with outside. Consequently there is no change in its internal energy according to the first law of thermodynamics. This means no change of temperature because the internal energy of an ideal gas depends only on temperature but not pressure or volume. It would be possible to restore the gas to its original state by compressing it isothermally. However, this entails doing work on the gas, which is achieved, for example, by a weight falling. There is then a change in the environment, so that the imagined process does not qualify for the consideration of irreversible transformation. 34 Reversible Transformations On the other hand, there are transformations of thermodynamic systems that are reversible. For example, the volume of a gas can be increased in a reversible isothermal process. To arrange for this, we place an ideal gas in a cylinder that has a piston on top and a bottom made of material that is perfectly heat conducting. The bottom sits on a large object with a fixed temperature. The piston is raised slowly to allow enough time for heat to flow from the large object so that the temperature of the gas remains fixed at that of the reservoir. The infinitesimal changes in volume add up to a sizable change. During the transformation, the state of the gas is described by its pressure, temperature, and volume that obey the ideal gas law. The process itself can be represented as a curve in the P-V diagram going from state A to B as shown: P A B V To go from B to A, we simply slowly lower the piston, allowing enough time for heat to flow out into the large object to maintain the same temperature. In general, in a reversible process, changes must occur very slowly so that there is time for the system to adjust to a state of thermal equilibrium. The process itself therefore goes through a succession of equilibrium states. A process for a thermodynamic system that starts from one state and ends with the same state is called a cyclic process. A cyclic process may or may not be reversible, because there is in general change in the environment. The recognition and analysis of irreversible transformations led to postulates with far reaching consequences. Postulate of the second law of dynamics (Kelvin Statement) A transformation whose only final result is to transform into work heat extracted from a source which is at the same temperature throughout is impossible. A source of heat that maintains the same temperature is called a heat reservoir. A device that can extract heat from a single heat reservoir and convert the heat 35 completely into work with no other change in the environment is called a perpetuum mobile of the second kind. (The first kind is one that generates work without the expenditure of energy or the absorption of heat, which is forbidden by the first law of thermodynamics). It does not violate the first law of thermodynamics. But the second law states that it does not exist. If it existed, it would be possible to draw on the practically infinite supply of heat from the ocean at no cost of materials. Heat Engines Fruitful analysis of the consequence of the postulate follows when we introduce a heat engine. This is a device employing a substance such as a fluid that can take an amount of heat from a hot reservoir, reject another amount to a cold reservoir, perform an amount of work on the environment, and return to the same state. The substance that has returned to the same state is said to undergo a cyclic process called a Carnot cycle. During one cycle of operation, if the amount of heat absorbed is Q1 , the amount rejected is Q2 , and the work performed is W , since there is no change in internal energy of the substance, application of the first law of thermodynamics yields U Q1 Q2 W 0 so that Q1 Q2 W A schematic diagram of a heat engine is as shown: Q1 W Q2 By definition, a reversible engine is one that can take Q2 back from the cold reservoir, have the work W done on the working substance by the environment, and delivers the heat Q1 back to the hot reservoir, while returning the substance to its original state. Not all engines are reversible. Consider two engines A and A’, with the corresponding heat and work in one cycle denoted by Q, W and Q’, W’ respectively. We can show: If A is reversible, then 36 Q1 Q1 Q2 Q2 If both A and A’ are reversible, then Q1 Q1 Q2 Q2 To prove the first relation, we first scale the engines so that Q2 Q2 . We run the engine A’ one cycle, followed by a reversed cycle of A (This is possible because A is reversible). If Q’1 > Q1, the amount of heat Q1 Q1 is extracted from the hot reservoir while no heat is exchanged from the colder reservoir in one combined cycle. Q’1 Q1 W’ Q’1-Q1 W =Q Q2=Q2’ Q’2 = Q Further, from the first law, Q1 Q2 W Q1 Q2 W , it follows that Q1 Q1 W W Thus, the amount of work done on the environment W’-W is positive. The net effect of the combined cycle is therefore to extract heat from one reservoir and perform an equal amount of work, while leaving no change anywhere. This is impossible according to the postulate of the second law of thermodynamics. Hence we must have Q1 Q1 0 37 W’-W =Q It then follows that Q1 Q1 , proving the first assertion. Q2 Q2 If A’ is also a reversible engine, by exchanging the roles of A and A’ in the forgoing argument, we have Q1 Q1 Q2 Q2 Taken together we prove the second assertion. The efficiency of a heat engine is defined as the fraction of heat absorbed that is converted into work. It is given by Q W Q1 Q2 1 2 Q1 Q1 Q1 Q2 is smaller for a reversible engine than an Q1 irreversible one, we conclude that all reversible engines operating between the same two heat reservoirs have the same efficiency, which is also larger than the efficiency of irreversible engines. Since we have shown that the ratio Carnot Cycle with Ideal Gas A reversible Carnot cycle can be realized using one mole of an ideal gas, operating between heat reservoirs at temperatures T1 and T2, measured in K. 38 The cycle starts with the gas in state a, at temperature T1 and volume Va. It is allowed to expand to the volume Vb in a reversible isothermal process to reach the state b. It is then further expanded to a volume Vc in a reversible adiabatic process to reach state c at the lower temperature T2. Then it is compressed isothermally to state d, with volume Vd and at temperature T2. An adiabatic compression then brings the gas back to state a. The heat absorbed from the reservoir at T1 during the isothermal expansion is V Q1 RT1 ln b Va The heat rejected to the colder heat reservoir during the isothermal compression is Q2 RT2 ln Vc Vd No heat is exchanged with the environment during the adiabatic processes, although both temperature and volume change would change. During the adiabatic expansion b to c, it can be shown that T1Vb 1 T2Vc 1 Similarly, during the adiabatic expansion d to a: T1Va 1 T2Vd 1 Taken together, we find Vb Vc V a Vd The expressions for Q1 ,Q2 from the isothermal processes therefore imply Q1 Q2 T1 T2 This shows that the efficiency of a reversible engine operating between heat reservoirs of temperatures T1 and T2 is given by 1 Q2 T 1 2 Q1 T1 39 A large efficiency requires a high value of T1 and a low value of T2. Normally, the lowest temperature we easily achieve is room temperature. So the only way to raise efficiency is to raise the temperature T1. For example, in a steam engine, the high temperature is 100°C so that T1=273+100=373K Taking the air temperature to be 20ºC, or T2=273+20=293K, the ideal efficiency is 1 293 21% 373 For an internal combustion engine, the heated gas can reach 800°C, or 1073K. The ideal efficient is 1 293 73% 1073 In practice, the efficiency is much less. Entropy For a substance undergoing one Carnot cycle in a heat engine, absorbing the amount of heat Q1 from a reservoir of temperature T1, and rejecting the heat Q2 to a reservoir at temperature T2, we have T1 Q1 T2 Q2 which can be written as Q1 Q2 0 T1 T2 with the equality sign holding if the cycle is reversible. Notice that –Q2 can be considered as the heat absorbed by the substance. More generally, it can be shown that if a thermodynamic system undergoes a cyclic process in which a series of heat absorptions Q1 , Q2 , Q3 , take place from heat reservoirs of various temperatures T1 , T2 , T3 , . , then Q Q1 Q2 T T1 T2 0 any cyclic process with the equality sign holding if the process is reversible. 40 If the system undergoes a reversible transformation from state A to state B, the quantity B A Q T is independent of the path (consisting of a series of intermediate equilibrium states) Q taken, and therefore depends only on the states A and B. To prove this, let T I Q and be the values for two reversible paths connecting A and B. Starting from T II state A, following path I and the reverse of path II, we arrive back at A. Since during the reverse process of II, heat absorption is Q from the reservoir of temperature T where Q and T are for the forward process II, we have according to the reasoning above for a reversible cyclic process, I Q 0 Q T T II which proves our assertion. The result allows us to introduce a new state variable called the entropy. The change of entropy between states A and B is defined as B S S B S A A Q T reversible process from A to B where the sum is over a reversible path. For any process I from A to B, we can complete a cyclic process with a reversible process II from B to A. Applying the definition of entropy to the path II: A S A SB B Q T reversible process from B to A The inequality B A Q A Q 0 cyclic process from A back to A T T B for any cyclic process can be written 41 B A Q S A SB 0 T . so that B SB S A A Q T for any process from Ato B An isolated system is one that does not exchange heat with its environment. For such a system, the right hand side of the above equation is zero. Thus, entropy does not decrease in an isolated system, and in fact, tends to increase as real processes, not being ideal, are irreversible. Examples of entropy increase 1. Frictional Heating. In Joule’s experiment, water is heated by paddles driven by a falling weight. Consider the water and the falling weight together forming an isolated system. There is no change of entropy associated with the falling weight, but the entropy of the water increases. This is because the rise in temperature of the water can be achieved also by reversible heat flow in which the water is brought into contact with heat reservoirs of successively higher temperatures. 2. Conduction of heat from a hot object to a cold object. An ice cube at 0°C in a room at a higher temperature melts gradually while the temperature stays the same. Consider the air in the room and the ice cube to form an isolated system. The heat flow away from the air can be taken to be a reversible process, causing a change of entropy Q Troom of the air. The same quantity of heat Q is absorbed by the ice, although the process is not reversible. However, the same change in the state of the ice, namely, the melting of a certain amount of ice at the temperature 0°C, can be effected by the slow (reversible) absorption of the same amount of heat. The change in entropy of the ice is therefore Q Tice The net change of entropy is Q Q Troom Tice and is positive because Troom Tice 42 3. Free expansion of a gas. Consider a thermally insulated rigid container to be divided into two compartments, with an ideal gas on one side and vacuum on the other. Upon removal of the partition, the gas expands to fill the whole volume. According to the first law of thermodynamics, there is no change in internal energy of the gas, because the gas does no work in expanding into a vacuum, and there is no exchange of heat. Hence the temperature remains unchanged because the internal energy of an ideal gas depends only on temperature but not volume. The final state of the gas can also be arrived at starting from the initial state by a reversible isothermal expansion. During this process, the gas absorbs the amount of heat Vf Q nRT ln Vi where Vi and V f are its initial and final volumes. (This result was obtained when we considered applications of the first law to ideal gas processes.) Therefore, by definition, the change of entropy of the gas is Vf Q nR ln T Vi and is positive because V f Vi . The fact that the entropy of an isolated system always increases can be understood to define an arrow of time: the flow of time is in the direction of entropy increase. 43