Thermodynamics - cloudfront.net
... of each of these handbooks was then reviewed by these two groups. This approach has resulted in revised modular handbooks that contain sufficient detail such that each facility may adjust the content to fit their specific needs. Each handbook contains an abstract, a foreword, an overview, learning o ...
... of each of these handbooks was then reviewed by these two groups. This approach has resulted in revised modular handbooks that contain sufficient detail such that each facility may adjust the content to fit their specific needs. Each handbook contains an abstract, a foreword, an overview, learning o ...
Adiabatic decompression and melting of mantle rocks
... [9] McKenzie [1984] assumed that CSp = Cm p = Cp. In that case, DCp,f = 0, and the terms within the first square brackets in the right of the above equation is the same as that derived by him [McKenzie, 1984, equation (D7)] for isentropic melting, (dx/dP)S, except that he had TDSf instead of DHf. No ...
... [9] McKenzie [1984] assumed that CSp = Cm p = Cp. In that case, DCp,f = 0, and the terms within the first square brackets in the right of the above equation is the same as that derived by him [McKenzie, 1984, equation (D7)] for isentropic melting, (dx/dP)S, except that he had TDSf instead of DHf. No ...
momentum the object has because it is spinning. (2) The other part
... things, so there is no useful relationship between them. The sign of the torque must be found by physical inspection of the case at hand. From the equation, we see that the units of torque can be written as newtons multiplied by meters. Metric torque wrenches are calibrated in N·m, but American ones ...
... things, so there is no useful relationship between them. The sign of the torque must be found by physical inspection of the case at hand. From the equation, we see that the units of torque can be written as newtons multiplied by meters. Metric torque wrenches are calibrated in N·m, but American ones ...
RIVAL™ RV500™ Polyurethane Clearcoat
... applying clearcoat. They should be lightly scuff-sanded if allowed to dry for more than 24 hours prior to application of RV500 clearcoat. Apply using a cross-coat technique - a wet coat using a top-to-bottom motion and a medium-wet second coat using a side-to-side motion. Flash 30 seconds to 5 min ...
... applying clearcoat. They should be lightly scuff-sanded if allowed to dry for more than 24 hours prior to application of RV500 clearcoat. Apply using a cross-coat technique - a wet coat using a top-to-bottom motion and a medium-wet second coat using a side-to-side motion. Flash 30 seconds to 5 min ...
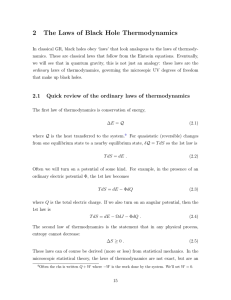
boltzmann`s entropy and time`s arrow
... Notions of probability Boltzmann's analysis implies the existence of a relation between phase space volume and probability. In particular, his explanation of the second law depends upon identifying a small fraction of the phase space volume with small probability. This is in the spirit of (but does ...
... Notions of probability Boltzmann's analysis implies the existence of a relation between phase space volume and probability. In particular, his explanation of the second law depends upon identifying a small fraction of the phase space volume with small probability. This is in the spirit of (but does ...
BOLTZMANN`S ENTROPY AND TIME`S ARROW
... Notions of probability Boltzmann's analysis implies the existence of a relation between phase space volume and probability. In particular, his explanation of the second law depends upon identifying a small fraction of the phase space volume with small probability. This is in the spirit of (but does ...
... Notions of probability Boltzmann's analysis implies the existence of a relation between phase space volume and probability. In particular, his explanation of the second law depends upon identifying a small fraction of the phase space volume with small probability. This is in the spirit of (but does ...
CHEM 155: BASIC PHYSICAL CHEMISTRY I INTRODUCTION
... 1. The gas is composed of a large number of identical molecules moving in random directions, separated by distances that are large compared with their size; 2. The molecules undergo perfectly elastic collisions (no energy loss) with each other and with the walls of the container, but otherwise do no ...
... 1. The gas is composed of a large number of identical molecules moving in random directions, separated by distances that are large compared with their size; 2. The molecules undergo perfectly elastic collisions (no energy loss) with each other and with the walls of the container, but otherwise do no ...
Book 5
... connected to a thin tube. When the bulb is immersed in a mixture of ice and water, the level of mercury in the tube is marked off and labeled 0°C. When it is immersed in a mixture of steam and water, the corresponding mark is labeled 100°C. The reason for choosing such mixtures to define 0°C and 100 ...
... connected to a thin tube. When the bulb is immersed in a mixture of ice and water, the level of mercury in the tube is marked off and labeled 0°C. When it is immersed in a mixture of steam and water, the corresponding mark is labeled 100°C. The reason for choosing such mixtures to define 0°C and 100 ...
An engine operates with 1 mol
... statement of the second law of thermodynamics is false, and show how this engine combined with a perfect refrigerator can violate the heat-engine statement of the second law. For this engine, Qh = 200 J, W = 60 J, and Qc = −140 J. A “perfect” refrigerator would transfer 140 J from the cold reservoir ...
... statement of the second law of thermodynamics is false, and show how this engine combined with a perfect refrigerator can violate the heat-engine statement of the second law. For this engine, Qh = 200 J, W = 60 J, and Qc = −140 J. A “perfect” refrigerator would transfer 140 J from the cold reservoir ...
Ideal Gases - fixurscore
... Kinetic theory of gases is a theory which links these microscopic properties (mass, velocity, kinetic energy) of particles to the macroscopic properties of a gas. On the basis of these assumptions, it is possible to use Newtonian mechanics to show the gas laws,…gas particles move with a range of spe ...
... Kinetic theory of gases is a theory which links these microscopic properties (mass, velocity, kinetic energy) of particles to the macroscopic properties of a gas. On the basis of these assumptions, it is possible to use Newtonian mechanics to show the gas laws,…gas particles move with a range of spe ...
Table of Content
... 0.0061 bar), with ice, water and steam co-existing. A system involving one pure substance is an example of a single-component system. On the other hand mixtures of water and acetone have two chemically independent components. ...
... 0.0061 bar), with ice, water and steam co-existing. A system involving one pure substance is an example of a single-component system. On the other hand mixtures of water and acetone have two chemically independent components. ...
Microfluidic mixing via transverse electrokinetic effects in a planar microchannel
... In recent years there has been a remarkable growth in research directed toward analytical devices on the micron scale. Lab-on-a-chip technology has the promise to fully integrate all stages of an analytical process, including chemical synthesis, characterization, separation, and detection within the ...
... In recent years there has been a remarkable growth in research directed toward analytical devices on the micron scale. Lab-on-a-chip technology has the promise to fully integrate all stages of an analytical process, including chemical synthesis, characterization, separation, and detection within the ...
MOLECULAR DYNAMICS BY COMPUTER SIMULATION (*)
... equations of motion for the molecules in the sample. Time averages are then calculated over those trajectories. MC methods 13-4 1 generate statistical ensembles by giving random displacements to the molecules and accepting, or rejecting, the resulting configurations with a probability proportional t ...
... equations of motion for the molecules in the sample. Time averages are then calculated over those trajectories. MC methods 13-4 1 generate statistical ensembles by giving random displacements to the molecules and accepting, or rejecting, the resulting configurations with a probability proportional t ...
V - people.vcu.edu
... Note: When a gas is changed from VI and PI to V2 and P2 at constant T it can be done many ways, and the W values obtained may be different for any two of these ways. • The gas may be expanded along a reversible path. This is indeed the path for which the integral of Pext dV for the change in state ...
... Note: When a gas is changed from VI and PI to V2 and P2 at constant T it can be done many ways, and the W values obtained may be different for any two of these ways. • The gas may be expanded along a reversible path. This is indeed the path for which the integral of Pext dV for the change in state ...
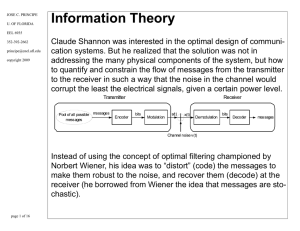
Information Theory
... and can also be thought as the gain of information. Note that if xk,yi are independent p(xk|yi)=0, that means we do not know anything about xk by observing yi. On the other hand if they are perfectly correlated, we know everything about xk (conditional probability is 1) and the mutual information de ...
... and can also be thought as the gain of information. Note that if xk,yi are independent p(xk|yi)=0, that means we do not know anything about xk by observing yi. On the other hand if they are perfectly correlated, we know everything about xk (conditional probability is 1) and the mutual information de ...
PDF
... vacuum state minimizes the output entropies for our isotropic Gaussian noise channels. Minimum output entropy corresponds to maximum output-state coherence. Physically, it does not seem possible that any non-vacuum state could extract coherence from the thermal-noise reservoirs associated with the t ...
... vacuum state minimizes the output entropies for our isotropic Gaussian noise channels. Minimum output entropy corresponds to maximum output-state coherence. Physically, it does not seem possible that any non-vacuum state could extract coherence from the thermal-noise reservoirs associated with the t ...
K - Rapid Learning Center
... (D) Would have less interactions between the particles and would cause more frequent collisions with the container and raise pressure, which would raise the volume if constant pressure and temperature are held constant. (E) Would increase the volume as the gas expanded to lower the internal pressure ...
... (D) Would have less interactions between the particles and would cause more frequent collisions with the container and raise pressure, which would raise the volume if constant pressure and temperature are held constant. (E) Would increase the volume as the gas expanded to lower the internal pressure ...
University of Maryland Department of Computer Science TR-4901
... occur. We chose the diffusivity coefficient, D = 1.8 × 10−9 cm s , which represents ∼ 3 µm particles in an aqueous solution. This value was used in [10]. It corresponds to a relatively small diffusivity constant, so that the problem is convection-dominated. A (mildly diffusive) model of this type cr ...
... occur. We chose the diffusivity coefficient, D = 1.8 × 10−9 cm s , which represents ∼ 3 µm particles in an aqueous solution. This value was used in [10]. It corresponds to a relatively small diffusivity constant, so that the problem is convection-dominated. A (mildly diffusive) model of this type cr ...
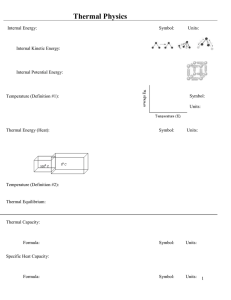
Thermal Physics - Physics Lectures
... Heat and work are ways of change the energy of the system. “Equilibrium” is recognised operationally as the circumstance under which bodies have ceased changing their physical state or condition. Heat is transferred between two systems when they come to thermal equilibrium without doing work o ...
... Heat and work are ways of change the energy of the system. “Equilibrium” is recognised operationally as the circumstance under which bodies have ceased changing their physical state or condition. Heat is transferred between two systems when they come to thermal equilibrium without doing work o ...
Powerpoint - University of Pittsburgh
... The second paper is a determination of the true sizes of atoms from the diffusion and the viscosity of dilute solutions of neutral substances. The third proves that, on the assumption of the molecular kinetic theory of heat, bodies on the order of magnitude 1/1000 mm, suspended in liquids, must alre ...
... The second paper is a determination of the true sizes of atoms from the diffusion and the viscosity of dilute solutions of neutral substances. The third proves that, on the assumption of the molecular kinetic theory of heat, bodies on the order of magnitude 1/1000 mm, suspended in liquids, must alre ...
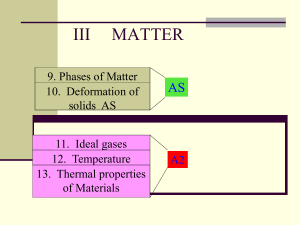
unit 9: thermal physics
... 1. Macroscopic behavior: Ideal gases increase in pressure when more gas is added to the container. Microscopic explanation: More gas means more gas particles in the container so there will be an increase in the number of collisions with the walls in a given interval of time. The force from each part ...
... 1. Macroscopic behavior: Ideal gases increase in pressure when more gas is added to the container. Microscopic explanation: More gas means more gas particles in the container so there will be an increase in the number of collisions with the walls in a given interval of time. The force from each part ...
Problem solving techniques: comparing two cases
... The ideal gas law applies: PV = nRT , but we don’t know either the volume or the number of moles of gas. So direct calculation of the pressure this way is not possible. Approach 1 : isolate the varying quantities, set the resulting expression in one case equal to the expression for the other case. S ...
... The ideal gas law applies: PV = nRT , but we don’t know either the volume or the number of moles of gas. So direct calculation of the pressure this way is not possible. Approach 1 : isolate the varying quantities, set the resulting expression in one case equal to the expression for the other case. S ...