* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download (1) - PhUSE Wiki
Effect size wikipedia , lookup
Plateau principle wikipedia , lookup
Drug discovery wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Version 1.0
1 Analysis and Displays associated with the
QT studies
Version 1.0 Draft for Broad Review
Created 08 September 2015
A White Paper by the PhUSE CSS Development of Standard Scripts
for Analysis and Programming Working Group
Disclaimer: The opinions expressed in this document are those of the authors and do not necessarily represent the
opinions of PhUSE, the members’ respective companies or organizations, or regulatory authorities. The content in
this document should not be interpreted as a data standard and/or information required by regulatory authorities.
1
Version 1.0
2 Table of Contents
1
Analysis and Displays associated with the QT studies ..........................................................................................1
2
Table of contents ...................................................................................................................................................2
3
Purpose ..................................................................................................................................................................3
4
Introduction ...........................................................................................................................................................4
5
ECG background ...................................................................................................................................................5
6
Pre-analytical issues ..............................................................................................................................................7
6.1
Correction of the QT-interval for heart rate .................................................................................................7
6.1.1
Historical Population-Based Formula from a Historical Population ........................................................7
6.1.2
Population-Based Formula from the Population under Study .................................................................9
6.1.3
Individual-Based Formula (QTcI) ...........................................................................................................9
6.2
Thorough QT (TQT) Study Design ............................................................................................................ 10
6.2.1
Brief Background ................................................................................................................................... 10
6.2.2
Specific designs ..................................................................................................................................... 14
6.3
7
Baseline and Treatment Difference (Drug Effect) ..................................................................................... 16
6.3.1
Time-Matched Lead-in Day Baseline; Double-Delta Treatment Difference ......................................... 16
6.3.2
Time-Averaged Lead-in Day Baseline; Double-Delta Treatment Difference ....................................... 16
6.3.3
Predose Averaged Baseline; Double-Delta Treatment Difference......................................................... 17
Analysis ............................................................................................................................................................... 18
7.1
Primary analysis ......................................................................................................................................... 18
7.1.1
Testing of QT prolongation ................................................................................................................... 18
7.1.2
Assay Sensitivity ................................................................................................................................... 19
7.1.3
Categorical Analyses ............................................................................................................................. 20
7.1.4
Morphological (Qualitative) Analyses ................................................................................................... 21
7.2
Concentration-Response Relationship (CRR) ............................................................................................ 21
7.3
P-values and Confidence Intervals ............................................................................................................ 23
8
List of outputs ...................................................................................................................................................... 24
9
Outputs shells ...................................................................................................................................................... 25
10
References ...................................................................................................................................................... 46
2
Version 1.0
3 Purpose
Under CDISC, standards have been defined for data collection (Clinical Data Acquisition Standards Harmonization
- CDASH), tabulation (Study Data Tabulation Model - SDTM), and analysis (Analysis Data Model - ADaM)
datasets. The next step is to develop standard tables, figures, and listings. The Development of Standard Scripts for
Analysis and Programming Working Group is leading an effort to create several White Papers providing
recommended analyses and displays for common measurements, and has developed a Script Repository as a place to
store shared code.
The purpose of this White Paper is to provide advice on displaying, summarizing, and analyzing Clinical Evaluation
of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs (henceforth referred to
as TQT study). The intent is to begin the process of developing industry standards with respect to analyses and
reporting for these trials. In particular, this White Paper provides recommended processes for:
Pre-analytical issues: Study design, QT interval corrections, and Baseline adjustments
Analytical issues: Testing for QT prolongation, Assay sensitivity, Outlier analysis / Categorical analysis,
Morphological (Qualitative) abnormalities, and PK/PD analysis
This paper attempts to give recommendations for difficult decisions related to the analysis of difficult topics such as
QT interval correction, baseline, and PK/PD analysis. Since there are on-going discussions regarding these topics
the recommendations made here are mainly based on the authors experience with these trials and submission to
regulatory bodies (and ICH-E14 guidelines and Q&A at the time this White Paper was written).
The content of this document can be used when developing the analysis plan for individual clinical trials for Clinical
Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs.
Development of standard Tables, Figures, and Listings (TFLs) and associated analyses will lead to improved
standardization from collection through data storage, as it is necessary to determine how the results should be
reported and analyzed before finalizing how to collect and store the data. The development of standard TFLs will
also lead to improved product lifecycle management by ensuring reviewers receive the desired analyses for
consistent and efficient evaluation of patient safety. Although having standard TFLs is an ultimate goal, this White
Paper reflects recommendations only and should not be interpreted as “required” by any regulatory agency.
Detailed specifications for TFL or dataset development are considered out-of-scope for this White Paper. However,
the hope is that specifications and code (utilizing SDTM and ADaM structures) will be developed consistent with
the concepts outlined in this White Paper, and placed in the publicly available Standard Scripts Repository.
3
Version 1.0
4 Introduction
Industry standards have evolved over time for data collection (CDASH), observed data (SDTM), and analysis
datasets (ADaM). There is now recognition that the next step would be to develop standard TFLs for common
measurements across clinical trials and therapeutic areas. Having industry standards for data collection and analysis
datasets provides a good basis for creating standard TFLs.
The beginning of the effort leading to this white paper came from the initiation of the FDA/PhUSE Computational
Science Collaboration, a yearly conference and ongoing working groups to support addressing computational needs
of the industry. The FDA identified key priorities and teamed up with the PhUSE to tackle various challenges using
collaboration, crowd sourcing, and innovation (Rosario LA, 2012). The FDA and PhUSE created several
Computational Science (CS) working groups to address several of these challenges. The working group, titled
“Development of Standard Scripts for Analysis and Programming,” has led the development of this white paper,
along with the development of a platform for storing shared code.
Several existing documents contain suggested TFLs for common measurements. Some of the documents are now
relatively outdated, and generally lack sufficient detail to be used as support for the entire standardization effort.
Nevertheless, these documents were used as a starting point in the development of this White Paper. The documents
include:
ICH E3: Structure and Content of Clinical Study Reports
Guideline for Industry: Structure and Content of Clinical Study Reports
Guidance for Industry: Premarketing Risk Assessment
Reviewer Guidance. Conducting a Clinical Safety Review of a New Product Application and Preparing a
report on the Review.
ICH M4E: Common Technical Document for the Registration of Pharmaceuticals for Human Use –
Efficacy
ICH E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential For NonAntiarrhythmic Drugs
ICH E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Nonantiarrhythmic drugs Questions and Answers R1.
FDA Guidance for Industry: ICH E14 Clinical Evaluation of QT/QTc. Interval Prolongation and
Proarrhythmic Potential for Non-Antiarrhythmic Drugs.
QT Studies Therapeutic Area Data Standards User Guide (TAUG) V1. CDISC.
The ICH E14 guidelines, FDA Guidance for Industry and TAUG are considered key documents. They do not
provide, however, detailed information that would enable standardization of all analysis and presentation of TQT
studies.
4
Version 1.0
5 ECG background
Some basic understanding of ECGs can be helpful in planning and completing analyses for Thorough QT (TQT)
studies. The ECG is a graphical representation of the electrical depolarization and repolarization of the heart’s cells
that initiates and spreads through the heart in an organized manner and causes contraction of the heart muscle that
results in the pumping of blood. In 1895, Einthoven established the five primary topographic features of the ECG
tracing (P, Q, R, S, and T waves; discussed in more detail below) and in 1912 defined the now standard ECG leads
(the waveform of potential difference over time between two sets of one or more electrodes attached to the body) I,
II, and III. Additional standard leads were established in 1938 (V1 – V6) and in 1942 (aVR, aVL, and aVF).
Therefore, the standard ECG records this activity at the body surface for 12 leads (I, II, III, aVR, aVL, aVF, V1, V2,
V3, V4, V5, V6). A continuous waveform (positive and negative changes over time) of electrical activity is
recorded for each lead. A standard ECG is a 10-sec recording, but ECG data can be recorded and stored digitally for
any amount of time (limited only by storage media capacity). A standard paper ECG displays 3 1/3 seconds of each
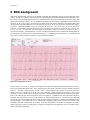
lead (4 sets of 3 leads) and all 10 seconds of 1 lead as illustrated in Figure 1.
Figure 5-1: Standard 10-sec ECG
The waveforms are a series of complexes that represent the sequential depolarization and repolarization electrical
activity that spreads through the heart. These complexes have parts, briefly noted above, that are named as shown in
Figure 2. Note that a single complex contains a P-wave, a QRS-complex (that consists of a Q-wave [sometimes
absent], an R-wave, and an S-wave; the R- and S-waves can have opposite polarities across leads), a T-wave, and
sometimes a U-wave. Each of these complexes represents a complete depolarization and repolarization of the heart.
There is an isoelectric gap (no electrical activity) between complexes. The RR-interval, not represented in Figure 2,
is the time between successive R-waves (and, therefore, the time between complexes). Analyses in TQT studies will
focus on The QT-interval and the RR-interval, but secondary analyses will also be conducted on the PR-interval and
the QRS-complex. The width of the waves, and intervals, including the RR-interval, represent time and are most
commonly expressed in millisecond (msec) units. Heart rate (HR) which is the number of complexes per minute,
usually expressed as beats per minute (bpm). Therefore, the RR-interval measurement, in msec, and HR, in bpm,
have the following relationship:
5
Version 1.0
RR = (1/(HR/60)*1000)
HR = 60,000/RR
Figure 5-2: A single ECG waveform complex and its parts
6
Version 1.0
6 Pre-analytical issues
6.1 Correction of the QT-interval for heart rate
The QT-interval is a measure or biomarker for the time of ventricular depolarization and repolarization to occur but
is practically used as a biomarker for the time of ventricular repolarization. The QT-interval changes in inverse
relationship to HR for appropriate physiological coordination of the pumping of blood by the heart. Therefore,
because subjects’ heart rates are not constant throughout participation in a TQT study (or when evaluated clinically)
it is necessary to correct the QT-interval for HR in order to make comparisons of the QT interval recorded at
different HRs at different times. Complicating the situation a bit more is the fact that the QT-interval does not
change instantaneously with a change in HR. The change in QT-interval is delayed; its change is subject to
hysteresis.
Hysteresis is generally ignored in the analysis of TQT studies, but one researcher (Malik, 2008) has developed
methods for evaluating hysteresis patterns on an individual basis and incorporating them into QT correction.
Discussion of this topic is beyond the scope of this White Paper.
The ideal corrected QT interval, QTc, would be uncorrelated with HR or the RR-interval. In other words if QTc
were plotted against either RR or HR and the data were fit to a linear model, the correlation would be “0” and the
slope of the regression line would be “0”. Essentially, QT correction for HR attempts to adjust the individual
subject’s QT-interval, at any HR, to a value that would be expected if the subject’s HR were constant. In the
majority of QT correction formulas, RR is used rather than HR because RR-interval is measured and expressed in
the same units as the QT-interval, msec, while HR is measured and expressed in bpm as illustrated above.
In general, there are three basic methods to adjust or correct the QT- interval for HR (RR-interval). The methods
are:
1. Historical population-based formulas derived from historical populations
2. Study population-based formulas derived from the populations under study
3. Individual-based formulas derived for each individual in the population under study
All three methods are based on exploring the mathematical relationship between the QT-interval and the RRinterval, but they use different populations for finding this relationship. The exploration of this mathematical
relationship amounts to finding a function and its numerical coefficients or finding the specific numerical
coefficient(s) for either a prespecified function or best fitting mathematical function (linear or nonlinear) from
among a number functions that models the relationship between the QT-interval and the RR-interval for a set of
ECGs from a population of multiple individuals (or from one individual in the case of Individual-based formulas).
The mathematical function is then translated into a correction formula using the numerical coefficient that was
found in the data fitting process. The same formula is then applied to all ECGs for which a QTc is being computed.
Therefore, for example, a set of QT-interval measurements and associated RR-interval measurements could be fitted
to the mathematical function:
QT = β * RRα
The value of the coefficient α that is found to give the best fit for the data might be 0.25. Then the correction
formula for QTc would be:
QTc = QT / RR0.25.
6.1.1 Historical Population-Based Formula from a Historical Population
In a historical population, this would be a group of normal, healthy persons, generally with 1 ECG from each person.
Due to the normal variance between different populations, multiple researchers using different groups of subjects
have derived different formulas even when fitting their respective data to the same mathematical function.
The most commonly used historical population-based correction formulae were proposed in 1920 by Bazett and
separately by Fridericia. Unfortunately, each formula can lead to bias for some clinically relevant values of HR as
will be illustrated below. For an extensive list of 31 such historical correction formulas, including those listed
7
Version 1.0
below, based on multiple mathematical functions, see a manuscript by Malik (2002). As indicated above, each of
these formulas could be expressed using HR, where RR miliseconds = ((1/(HRbeats-per-minute/60))*1000)
QTc = QT/ RR1/2
(i)
Bazett:
(ii)
Fridericia: QTc=QT/RR1/3
(iii)
Framingham: QTc=QT+(0.154*(1-RR))
(iv)
Van de Water: QTc=QT–((0.087*(1-RR))
It is reasonably well known that the Bazett formula under-corrects at faster heart rates (over 60 bpm) and conversely
over-corrects at slower heart rates. That is, at faster HRs (smaller R- intervals), the computed QTc is ‘larger than it
should be’ and at slower HRs (larger RR-intervals), the computed QTc is ‘smaller than it should be’. When Bazett
corrected QTc is plotted against RR interval and a regression line is plotted, the slope is negative (Figure 3; with a
perfect correction, the slope of the regression line would be “0” as described above). In spite of this, Bazett’s
formula is still the most widely used for clinical correction of QT intervals. However, it is becoming more
acceptable in regulatory documents to use the Fridericia formula correction, without use of the Bazett formula (ICH,
2012; Question 11), along with additional correction results as described below.
8
Version 1.0
Fridericia correction
Corrected QT interval (msec)
Corrected QT interval (msec)
Bazett correction
500
450
400
350
500
450
400
350
0.6
0.8
1.0
1.2
0.6
0.8
1.0
1.2
RR interval (sec)
RR interval (sec)
Figure 6-1: Relationship between the Bazett- and Fridericia-Corrected QT Interval and RR Interval
(Note that the solid line is not the linear regression line but the mean of QTc values at each RR value)
6.1.2 Study Population-Based Formula from the Population under Study
A study population formula derived from the population under study uses off-treatment, baseline ECGs, and
sometimes ECGs collected during placebo treatment to construct a population correction formula as described
above.
The method is based on finding the specific numerical coefficient(s) for either a prespecified or best fitting
mathematical function (linear or nonlinear) that models the relationship between the QT-interval and the-RR interval
for a set of ECGs from a population of multiple individuals. The mathematical function is then translated into a
correction formula using the numerical coefficient that was found in the data fitting process. The same formula is
then applied to all ECGs for which a QTc is being computed.
Because the formula is based on the behavior of the individuals actually under study, such a study populationderived formula presumably accounts for variables (e.g., disease factors, age, and gender distribution) which might
influence the QT-RR relationship. Therefore, such a formula should be more accurate for the individuals under
study than one based on a historical population.
6.1.3 Individual-Based Formula (QTcI)
It has been well established that the mathematical function that best describes the QT-RR relationship may differ
from individual to individual (Malik, 2002b) but is stable within individuals, and, therefore, any group-based (studywide) correction will be somewhat imprecise when applied to individuals. While the magnitude of imprecision is
generally not of sufficient magnitude to affect substantially negatively the TQT study, it is possible to derive and use
individual-based correction formulas. An individual-based QTc (QTcI) requires that a number of ECGs be obtained
across a sufficient range of HRs. The number of ECGs required for individual correction is an important matter.
Morganroth (2005) has suggested that 35 to 50 ECGs covering a range of heart rates of 50 to 80 beats per minute for
each individual under baseline (nontreatment) conditions are sufficient. Couderc (2005) has published data to
support the position that at least 400 ECGs (QT-RR pairs for each individual subject) are needed to compute an
adequate individual correction and that there must definitely be a range of heart rates corresponding to the heart
rates that will be observed with the experimental drug. These QT-RR data are then used to compute a specific
correction formula for each individual subject in a manner similar to that used to compute a population correction.
In computing QTcI, one sub-approach is to use a single, predetermined mathematical model for all subjects and we
can refer to this approach as individualized correction (optimizing the coefficient[s] on an individual basis for a
9
Version 1.0
single correction formula). An alternative sub-approach is to fit the individual subject’s data to several preselected
mathematical models and use the best mathematical model for each individual subject (model with the best fit to the
data and that results in flattest regression line after correction (QTcI vs. RR)) and we can refer to this approach as
individualized individual correction (optimizing the actual correction formula and its coefficient[s] on an individual
basis). Malik et al. (2004) have described 12 mathematical models that could be considered when finding an
individual best-fit model for a given subject. As such, this latter method for computing QTcI, individualized
individual correction, is probably the best. However, either type of individual correction formula computation is
also very labor intensive and costly to use.
Some researchers have developed methods of assessing changes in ventricular repolarization based on the QT
interval that do not rely on an explicit correction of the QT- interval for HR (the RR-interval). These methods are
particularly important when the experimental drug results in marked changes in autonomic nervous system tone and
HR. These changes can be so large that it will be difficult to obtain ECG data at heart rates that will be observed
during treatment with the experimental drug, which would raise concerns about the validity of any correction factor.
Discussion of these alternatives beyond the introduction of the concept is outside the scope of this document but can
be reviewed in the manuscript by Garnett et al. (2012). These methods would generally rely on continuous
recording data.
6.2 Thorough QT (TQT) Study Design
6.2.1 Brief Background
6.2.1.1 Historical Reason for the TQT Study
Jervell and Lange-Nielsen (1957) described correlations between hereditary long QT intervals and sudden death.
Smirk and Palmer (1960) noted that initiation of ventricular depolarization (R waves) prematurely occurring before
the complete repolarization of the ventricle following the preceding depolarization (during the T waves – referred to
as “R-on-T Pattern”) increase the risk of ventricular arrhythmia. Torsade de Pointe (TdP), a specific type of
ventricular tachyarrhythmia (fast arrhythmia), was first described in a publication by Dessertenne (1966).
Although some drugs that had been developed as anti-arrhythmic agents also altered ventricular repolarization as
evidenced by prolonged QTc, it was not widely appreciated that non-cardiac drugs could also have this property.
The use of non-sedating antihistamines, e.g. terfenadine and astemizole, from 1985 to 1999 provided an important
case study of the public health issues with the widespread use of non-cardiac drugs with such cardiac effects. Initial
reports of cardiac arrhythmias, including TdP, were predominately associated with high blood concentrations of
these antihistamines subsequent to overdose. Given the metabolic pathway of these drugs, arrhythmias were
eventually reported subsequent to co-administration with drugs and substances that slowed the metabolism of
terfenadine and astemizole, including grapefruit juice (also resulting in high blood concentrations). Despite warning
letters to physicians and restricted product labeling in 1992, inappropriate medications continued to be coadministered with these drugs. Both drugs were withdrawn in 1999 from use in the U.S. after safer alternatives were
developed.
The high visibility of the association between non-sedating antihistamines and fatal ventricular arrhythmias
prompted extensive research into the mechanisms by which drugs cause these cardiac arrhythmias. Although many
details remain unknown, current research suggests that most drugs with strong arrhythmic potential interfere with a
specific potassium channel in cardiac muscle fiber that functions to repolarize the muscle fiber cells. Partially or
completely blocking the potassium channel results in delayed repolarization of the muscle fiber cells. Delayed
repolarization increases the time required to restore the normal resting potential prior to the next depolarization for
the next muscle contraction. Arrhythmias such as TdP are possibly triggered by the initiation of the R-waves
(beginning the depolarization of the ventricles) during the period of delayed repolarization while the ventricles are
still partially depolarized. In summary, drugs that delay ventricular repolarization might place a person at increased
risk of a fatal ventricular arrhythmia.
Again, delayed ventricular repolarization is manifested on the ECG tracing as a prolonged QTc. QTc is clearly
recognized as an imperfect biomarker for increased risk of fatal arrhythmia because it can be increased by a number
10
Version 1.0
of drugs that are not associated with a significant incidence of such arrhythmias. None-the-less, an increase in QTc
is considered an important risk factor and any drug-induced increase is considered important to assess and quantify.
On an individual basis, the increase in QTc generally needs to be substantial to place the patient at risk, but for a
potential new drug, even a slight mean increase1 in QTc can be clinically meaningful, in that some degree of risk
cannot be excluded in a small number of individuals in a large population that will receive the drug during its use in
clinical medicine. The TQT study is considered the most precise way of studying the potential drug effect on QTc
in human subjects.
6.2.1.2 Study Design Background Considerations
Clinical studies to detect QTc mean increases as small as 5 msec face significant challenges because of the
substantial variability in QT intervals. The first source of variability is the process of acquiring and measuring the
QT interval. Placement of ECG electrodes, choice of lead(s) to be measured, standardization of ECG machines,
choice of media (paper vs. digital), and variability in expert measurement of the QT interval comprise critical
components of the process.
QT intervals are characterized by substantial inter- and intra-subject variability apart from that engendered by
acquisition and measurement. Sources of inter-subject variability can include a genetic predisposition to long QT
intervals, electrolyte concentrations, autonomic activity, age, and sex. Intra-subject variability is strongly influenced
by diurnal rhythms (transitioning to sleep from wakefulness and vice versa) that influence autonomic tone and heart
rate.
Dose selection, duration of dosing, timing of ECG measurements, patient population, and control of factors
influencing variability will need to be addressed in any study designed to evaluate QT interval. While the TQT
study is considered the most definitive study of the potential influence of a drug on QT interval, it might suffer from
limitations due to sample size, the health of the subject population, and many other factors that cause the drug
administration in the study to be different from how the drug will be used in broad clinical practice.
This brief background provided below is informed primarily by the May 2005 ICH-E14 document [ICH, 2005],
which describes the basic conduct, purpose, and expected analyses of the TQT study as well as its update in a
subsequent Q&A document (ICH, 2012)
The purpose of a TQT study is to evaluate the potential for an experimental drug to delay cardiac ventricular
repolarization, which the study does through evaluation of changes in QTc during drug treatment; and also to
demonstrate that the study is capable of detecting differences in the variability that can be observed during placebo
treatment (random variability; approximately 5 msec), so as to confirm that any lack of detected change is due to
actual lack of change rather than lack of assay sensitivity. These TQT studies are generally conducted in healthy
volunteers, highly screened for normal cardiac electrical activity, for ease of precise measurement of the QTinterval, and to avoid additional confounding factors.
The TQT study designs can be a crossover design or a parallel design discussed in more detail below. In general,
the treatments are:
1. A dose of the experimental drug that is several times higher, if possible, than the intended maximum dose,
in order to account for drug-drug interactions and/or genetic metabolic enzyme deficiencies that might lead
to greater exposure to the experimental drug than otherwise intended with a given dose during routine
clinical use
2. Placebo
3. A positive control for purpose of demonstration of assay sensitivity (most often moxifloxacin, usually oral
but sometimes intravenous)
4. Optionally, a dose of the experimental drug that is within the intended therapeutic range (generally the
maximum intended therapeutic dose)
1
>5 milliseconds (msec) would be considered to exceed random variability (Malik, 2001) and a mean increase of ≥10 msec could be of
regulatory interest (ICH, 2005).
11
Version 1.0
The administration of the active control has been allowed by regulators to be open-label, but the administrations of
the experimental drug dose(s) and placebo are double-blind, and ECG measurements and readings are performed by
persons completely blinded to associated treatments, subject details, and date/time of the ECG.
6.2.1.3 Days of ECG Collection and Time points of ECG Collection on the Days of
Collection
ECGs are collected as a set of replicates (in close temporal proximity, e.g., 3 ECGs collected at 1-minute intervals)
of 10 seconds in duration and utilizing all 12 leads. In analyses, the QTc values of the replicates will be averaged
before analysis of differences in changes in QTc to reduce the signal-to-noise ratio and improve the accuracy of the
measurement. When discussing the collection of ECGs below, “ECG” will refer to the set of replicate ECGs. ECGs
can be collected as conventional ECGs, or they can be extracted from a continuous high fidelity ECG recording.
Experimental drug and metabolite concentrations are often collected for assay immediately after the time of ECG
collection for PK/PD analysis, which can be a useful secondary analysis pertinent to the potential influence of the
experimental drug on ventricular repolarization.
The timing and collection of replicate ECGs are guided by the known properties (e.g., PK) of the drug and its
metabolites.
6.2.1.3.1 Baseline ECGs
In general, in the analyses of QTc, baseline QTc values are subtracted from on-treatment QTc values to create a
“single ∆” value that is “change in QTc” and this “change in QTc” is compared between treatments. Several
alternative baselines exist as will be further discussed in Section 4 below that describes alternative analyses that are
in large part influenced by the definition of baseline. Baseline ECGs are collected:
On the day (or for multiple days) preceding the day of first dose administration of each treatment;
If this type of baseline is used, ECGs are collected at multiple time points that match the
time points at which ECGs are collected on-treatment
If ECGs are collected on multiple days, then QTc values from those days can be averaged
for the baseline value used in analyses; this multiple baseline day collection is rarely done
The averaging can be for each time point when a time-matched baseline is being
used (time-matched) or across all time points (time averaged), if a time averaged
baseline is being used (see Section 4. Baseline and Treatment Difference below
for a more detailed description of baseline alternatives)
Baseline day(s) and time points are the same for each treatment to maintain blind
Although consideration might be given to using a single, common baseline for each
treatment in a crossover study, either before the first treatment period for all subjects or
with a subset of subjects assigned by random allocation before each of the treatment
periods (Section 3.2 discusses study design in more detail), this is not done
This baseline that collects multiple ECGs at the same time points as the ECGs will be
collected while on treatment on at least one day that precedes the first administration of
test is necessary for parallel studies (allows time-matched baseline)
and/or
Immediately preceding the first dose administration of each treatment
ECGs would be collected at several time points shortly before first dose administration
such as 60 minutes, 45 minutes, 30 minutes, 15 minutes, and immediately before
treatment administration
This baseline is generally used for crossover studies and not allowed by regulators for
parallel design studies
This baseline ECG collection can be combined with the ECG collection on the day or
days preceding treatment administration, resulting in complex baseline definitions and
treatment difference definitions
12
Version 1.0
6.2.1.3.2 On-treatment ECGs
The days on which ECGs are collected and the time points of collection are determined by the PK characteristics of
the test drug. The intent of the study is to measure QTc at that time at which a maximum increase in QTc would
occur if the drug, or relevant metabolites, does increase QTc. In crossover studies, it is often the case that the drug
is sufficiently well tolerated that desired supratherapeutic exposure could be achieved with a single dose, so only a
single dose of treatments is given. Sometimes in crossover studies, it is necessary to titrate the drug up to intended
exposure with multiple doses over multiple days. In parallel studies, dosing is often extended over multiple days
before intended exposure is reached.
For single-dose studies, ECGs are collected on the day of treatment administration at a time point shortly before the
time of the maximum drug concentration (T max), around Tmax, and should continue even after T max to evaluate any
delayed effects of the drug or its metabolites on cardiac repolarization. Depending on the PK of drug and
metabolites, the ECG collection might continue for one or more days following the day of drug administration.
For multiday dose studies, ECGs are collected according to the schedule described in the paragraph above but
beginning on the day that the drug reaches steady state or intended exposure has been achieved. In some multiday
dose studies, ECGs will also be collected following the first dose at identical times.
To demonstrate assay sensitivity, ECGs should also be collected close to the Tmax of the positive control.
Replicate ECGs should be collected on the same days and at the same time points in all treatment groups to ensure
that blinding is maintained.
The diagrams below show how ECG data are organized within 10-second ECGs, and how those 10-second ECGs
are organized within and across time points. Although analysis methods that use all the data from continuous
monitoring over a long period (e.g., 24 hours) have been developed, the analysis usually assumes that data is
organized by time points. ECGs should be recorded (or extracted from continuous recordings) in triplicate as noted
above (replicates, number can vary but will generally be 3 and can be more), 30-120 seconds apart, to account for
inherent variability; each recording lasting 10 seconds (these 10-sec ECGs are either recorded as 10-sec ECGs or
extracted from continuous recording of the ECG record that is digitally stored for later processing, typically in 24hour increments). Figure 5 is illustrating the on-treatment collection of triplicate ECGs, as an example of the
replicate collection, on a single day of ECG collection following treatment administration.
24
hours
Beat 1
(7:59:00.00)
Beat 2
(7:59:01.30)
Beat 3
(7:59:02.15)
… Beat 12
(7:59:10.05)
…
Beat
100,800
Extracted 10second ECG
12 P-QRS-T
Figure 6-2: Illustration of 1 of 12 Leads of Continuous ECG recording from which a 10-sec ECG can be
extracted
Each cycle, in a normal ECG obtained from a healthy person, consists of a P-QRS-T complex and the subsequent
isoelectric activity before the next P-QRS-T complex as described above in Section 1.
13
Version 1.0
Figure 6-3: Illustration of the concepts of recording multiple replicate ECGs at multiple time points
subsequent to treatment administration (recording would also occur at baseline)
ECGs are taken after subjects have rested, but not sleeping, for at least 5 to 10 minutes in the supine position (in an
attempt to obtain a stable heart rate under similar physiological conditions at each time of collection). If the ECGs
are to be extracted from a continuous recording, then the subjects rest as they would for actual 10-second ECG
recordings.
6.2.2 Specific designs
The examples of study designs presented below illustrate specific TQT study designs. A typical TQT study is
designed as double-blind (partial double-blind as in some cases the investigator might not be blind to administration
of the active control), placebo- and positive-controlled to determine whether the test treatment fails to prolong the
QTc (primary statistical test is noninferiority) and to demonstrate the assay sensitivity using the positive control
treatment in the study population. Traditional TQT studies employ parallel or crossover designs, are generally
designed with equal study duration, and sample size for the different treatment arms or periods.
6.2.2.1 Parallel Studies
Under certain circumstances (related to the PK characteristics of the test drug), a parallel design may be preferred
for a TQT study. Such circumstances include (ICH, 2005):
• Drugs with long elimination half-lives for which lengthy time intervals would be required to achieve
steady-state and complete washout
• If carryover effects are prominent for other reasons, such as irreversible receptor binding or long-lived
active metabolites
• If multiple doses of the investigational drug are required to evaluate the effect on QT/QTc intervals
14
Version 1.0
Example of TQT - Parallel Study
Below is the study schema diagram for a parallel study. This study has 4 treatment arms (placebo, positive control,
therapeutic study drug dose, and supratherapeutic study drug dose), which correspond to the 4 possible left-to-right
"paths" through the study. Moxifloxacin has become the standard positive control with a well characterized (peak
effect and time course), expected influence on QTc in healthy subjects with a mean increase in QTc in the range of
10 – 15 msec. Other positive control compounds are possible (e.g., low dose ibutilide).
Note: Moxifloxacin is one
example of a positive control.
Note: This is an optional arm.
Figure 6-4: Parallel Study Design Schema for Example TQT Study 1
T = Therapeutic Dose (DRUG A 1 MG), ST = Supratherapeutic Dose (DRUG A 100 MG)
6.2.2.2 Crossover Studies
In comparison to parallel studies, crossover studies have at least two potential advantages:
• A smaller number of subjects are typically required. Subjects serve as their own controls, resulting in
reduced variability of differences related to inter-subject variability.
• Heart rate correction approaches based on individual subject data may be more feasible (as baseline ECGs
are collected before each treatment period; therefore, more ECGs are available for each subject for
computation).
Example of TQT – Crossover Study
Below is the study schema diagram for a crossover study. In this example, subjects were screened for eligibility and
then randomized in a 1:1:1:1 ratio to receive 1 of 4 treatment sequences (Williams design). As with the parallel
design, the therapeutic dose is optional.
If the test drug is sufficiently well tolerated such that the necessary supratherapeutic exposure can be achieved with
a single dose and washout is not lengthy, then these crossover studies often involve administration of a single dose
of drug. If the drug must be titrated to reach required exposure but that titration period is not too lengthy, and
washout is not lengthy then the crossover design can be used. When that titration or washout is lengthy, the parallel
design is used. Sponsors make the decision regarding whether a study should be crossover or parallel based on the
required titration and or washout time.
As in most crossover studies, the treatment arms are distinguished by the order of treatments, with all treatments
present in each arm.
Figure 6-5: Crossover Study Design Schema for Example TQT Study 2
T = Therapeutic Dose (DRUG A 1 mg), ST = Supratherapeutic Dose (DRUG A 100 mg)
A washout period sufficient to clear all drug exposure would be present between treatment periods.
15
Version 1.0
6.2.2.3 Non-standard designs
A design has been used for a parallel TQT study that required lengthy treatment periods in which the positive
control treatment was embedded in the placebo treatment arm (Malik, 2008b). Discussion of this design alternative
is beyond the scope of this White Paper, but the reader can review the cited manuscript.
When both a therapeutic dose and a supratherapeutic dose are studied, they might be contained in a single arm of a
parallel study with the supratherapeutic dose following the therapeutic dose (dose escalation) or the supratherapeutic
dose can follow the therapeutic dose (dose escalation) in a crossover study. When such designs are employed, the
supratherapeutic dose clearly does not have the same design characteristics as the other treatments and questions
regarding potential bias can arise. Discussion of such design alternatives is beyond the scope of this White Paper.
6.3 Baseline and Treatment Difference (Drug Effect)
In this section, three different baseline definition alternatives are described. For each baseline definition, the
resulting definition of treatment differences is described. Note that this is not an exhaustive list of possibilities. For
example, triple-delta (∆∆∆QTc) treatment difference definitions are possible where both lead-in day ECGs are
collected at matched time points to the time points of collection on the treatment day and one or more ECGs are
collected immediately before treatment administration (and at the same time point on the lead-in days), essentially
combining 6.3.1 and 6.3.3 below. Multiple lead-in days could be used to create averaged lead-in day values to be
used for a time-matched baseline. Potentially, on-treatment QTc values could be compared without any baseline
difference comparison, especially in crossover studies where each subject is acting as his/her own control (singledelta - ∆QTc).
6.3.1 Time-Matched Lead-in Day Baseline; Double-Delta Treatment
Difference
For time-matched baseline, the baseline for each period is the average of the replicate set values at a time point on
the lead-in (baseline) day (Day -1) that corresponds to the post-dose time point. ECGs are collected or extracted
from continuous recording in replicate sets (usually 3 replicates about a minute or so apart) at each b j and Xij. The
average of the replicates is used for analysis. With the original ICH E-14 guidance, this was the standard baseline
definition for both crossover and parallel studies. With the publication of the ICH E-14 Q&A (ICH, 2012; Question
6), the requirement for this baseline definition for crossover studies was relaxed (See Section 6.3.3 below)
For crossover design, ΔΔQTcij is computed for each subject: ΔΔQTcij = (Xij − bj )
Drug A
− (Xij − bj )
Placebo
where
i=1, 2, … d, j=1, 2, … n; d=days postdose and n=time point. ΔΔQTcij is the difference between drug and placebo in
the change from baseline (time-matched) in QTc at each time point for each day of treatment on an individual
subject basis.
̅̅̅̅̅̅̅̅̅̅
̅̅̅̅̅̅̅̅̅̅
For a parallel design, (Xij – bj) would be averaged across subjects: ΔΔQTcij = (X
− (X
ij − bj )
ij − bj )
Drug A
Placebo
ΔΔQTcij is the difference between drug and placebo in the average across subjects of the change from baseline
(time-matched) in QTc at each time point for each day of treatment.
6.3.2 Time-Averaged Lead-in Day Baseline; Double-Delta Treatment
Difference
For time-averaged baseline from a lead-in (baseline) day, the baseline for each period is the average of all values for
all the replicate sets of ECGs on the baseline day (e.g. Day -1, 1 hr., 2 hr., 3 hr., 4 hr., etc.). ECGs are collected or
16
Version 1.0
extracted from continuous recording in replicate sets (usually 3 replicates about a minute or so apart) at each b j and
Xij. The average of the replicates is used for analysis. Several statistical manuscripts have advocated this baseline
definition over the time-matched lead-in day baseline for parallel studies (Meng, 2010) and both crossover and
parallel studies (Sethuraman, 2008) but this has not become a regulatory standard.
For crossover design, ΔΔQTcij is computed for each subject: ∆∆QTcij = (Xij − bavg )
Drug A
− (Xij − bavg )
Placebo
where bavg = ∑ bj /n; i=1, 2, … d, j=1, 2, … n; d = days postdose and n = time point. ΔΔQTcij is the difference
between drug and placebo in the change from baseline (time-averaged) in QTc at each time point for each day of
treatment on an individual subject basis.
̅̅̅̅̅̅̅̅̅̅̅̅̅
For a parallel design, (Xij – bavg) would be averaged across subjects: ΔΔQTcij = (X
ij − bavg )Drug A −
̅̅̅̅̅̅̅̅̅̅̅̅̅
(X
ij − bavg )Placebo ΔΔQTcij is the difference between drug and placebo in the average across subjects of the
change from baseline (time-averaged) in QTc at each time point for each day of treatment.
6.3.3 Predose Averaged Baseline; Double-Delta Treatment Difference
For predose averaged baseline, ECGs are collected or extracted as replicate sets (usually 3 replicates about a minute
or less apart) at predose in close temporal proximity to treatment administration (e.g., 15 min intervals and
immediately before treatment administration on the same day of treatment administration) and as replicate sets
(usually 3 replicates about a minute or so apart) at each X ij post dose. The average of all the replicates collected
predose is used as the baseline for analysis. This baseline definition has the advantage of eliminating the necessity
of an inpatient lead-in day from the experimental design with all the monetary and operational expense for each
treatment period. This baseline definition is now accepted for crossover studies that are both single-dose and
multiple-dose administered over multiple days (ICH, 2012; Question 6).
For crossover design, ΔΔQTcij is computed for each subject: ∆∆QTcij = (Xij − b0 )
Drug A
− (Xij − b0 )
Placebo
where
b0 = ∑ bj /k; i=1, 2, … d, j=1, 2, … n; d = days postdose, k = number of predose replicates, n = time point. ΔΔQTcij
is the difference between drug and placebo in the change from baseline (predose-matched) in QTc at each time point
for each day of treatment on an individual subject basis.
̅̅̅̅̅̅̅̅̅̅̅
For a parallel design, the (Xij – bj)’s would be averaged across subjects: 𝛥𝛥𝑄𝑇𝑐𝑖𝑗 = (𝑋
𝑖𝑗 − 𝑏0 )𝐷𝑟𝑢𝑔 𝐴 −
̅̅̅̅̅̅̅̅̅̅̅
(𝑋𝑖𝑗 − 𝑏0 )𝑃𝑙𝑎𝑐𝑒𝑏𝑜 ΔΔQTcij is the difference between drug and placebo in the average across subjects of the change
from baseline (predose-averaged) in QTc at each time point for each day of treatment.
17
Version 1.0
7 Analysis
7.1 Primary analysis
There are two hypothesis tests to be performed in a thorough QT/QTc studies:
1. The hypothesis test to confirm no study drug effect that results in prolongation of the QT/QTc as compared
to the placebo group;
2. The study is capable of detecting differences in QT/QTc, however, small it may be (to establish the assay
sensitivity) by demonstrating the QT/QTc effects of the active control that are already known.
7.1.1 Testing of QT prolongation
The primary endpoint should be the time-matched mean difference between the drug and placebo after baseline
adjustment at each time point.
According to the ICH E14 (Section 2.2.4), the test drug is classified as negative (lack of evidence of QT/QTc
prolongation) if the upper bound of the one-sided 95% confidence interval for the largest time matched mean
difference between the drug and placebo excludes 10 msec. This definition was chosen to provide reasonable
assurance that the mean effect of the study drug on the QT/QTc is not greater than around 5 ms. When the largest
time-matched difference exceeds the threshold, the study is termed “positive,” (lack of prolongation effect cannot be
established) and additional electrocardiogram safety evaluation in subsequent clinical studies should be performed.
The QT intervals (means of replicates) are usually measured at multiple time points to provide reasonable assurance
that the mean difference between study drug and placebo on the QT/QTc interval is not greater than the pre-defined
threshold. In practice, an intersection-union test (IUT) is applied for its practicality, ease of implementation, and
conservatism with respect to assessing QT/QTc prolongation. It is the uniformly most powerful unbiased test
(Berger and Hsu, 1996). The hypothesis is specified as follows:
T
H0T : {(drug(
ti ) placebo (ti ) ) 10}, i 1, 2,..., n , versus
T
H1T : {(drug(
ti ) placebo ( ti ) ) 10}, i 1, 2,..., n
where
T
drug(
t)
i
and
placebo(t ) are the mean change from baseline of QT for drug and placebo respectively, at time
i
point ti.
The statistical model for estimating the treatment effects and the confidence intervals depend on the study design
and other factors. An analysis of covariance model (ANCOVA) or repeated measures mixed effects model is
usually used to estimate the treatment effect and the confidence intervals. For crossover designs, the ANCOVA
model usually includes treatment, time, period, sequence, and the time-by-treatment interactions as fixed effects, and
“pre-Dose averaged” baseline as a covariate. For parallel designs, the model usually includes treatment, time as
fixed effects, and “Time-Matched” baseline as a covariate. The ANCOVA model using day-averaged (timeaveraged; 6.3.2 above) baseline is recommended for the analysis of parallel-group thorough QT/QTc studies (Sun
and Quan etc. 2012). Other covariates should only be added in an exploratory fashion (the simpler model being the
primary analysis) only if there are excellent clinical reasons for including them.
7.1.1.1 Multiplicity Issues
For the test drug to placebo comparison, as noted above, an intersection-union test (IUT) method has been proposed
and most frequently used as the primary method of analyzing the through QT/QTc study.
The IUT method controls the Type I error. Specifically, the comparison between the test drug and placebo requires
no adjustment for multiplicity and thus the standard one-sided 95% confidence intervals are used at all post-dose
18
Version 1.0
time points. However, the IUT method may potentially lead to false positive trial results (failing to reject inferiority
[not finding non-inferiority] in this specific case of TQT study analysis). The false positive rates depend on several
factors including variability of the study, sample size, the number of time points, and the true mean difference to be
detected. The probability of incorrectly not being able to reject a potentially clinically meaningful QT/QTc effect
increases (or statistical power decreases) with the number of post-dose time points (Patterson et al., 2005).
7.1.2 Assay Sensitivity
The confidence in the ability of the study to detect QT/QTc prolongation can be greatly enhanced by the use of a
concurrent positive control group to establish assay sensitivity. The positive control should have an effect on the
mean QT/QTc interval of about 5 ms (i.e., an effect that is close to the QT/QTc effect that represents the threshold
of regulatory concern, around 5 ms). However, as moxifloxacin is the accepted regulatory positive control standard,
an effect in the 10-15 ms range for the positive control is acceptable.
In the ICH E14 Question and Answers in 2012 [1], FDA clarified how to access the adequacy of the positive control
in the QTc study. There are two conditions required for ensuring assay sensitivity:
1.
The positive control should show a significant increase in QTc; i.e., the lower bound of the one-sided 95%
confidence interval (CI) must be above 0 ms. This result shows that the trial is capable of detecting an
increase in QTc, a conclusion that is essential to concluding that a negative finding for the test drug is
meaningful.
2.
The study should be able to detect an effect of about 5 ms (the QTc threshold of regulatory concern).
Therefore, the size of the effect of the positive control is of particular relevance. It determines the
threshold of the lower bound.
a.
If a positive control has a known effect of greater than 5 ms (e.g., 10 ms), assay sensitivity will be
established if the lower bound of the one-sided 95% confidence interval for the mean treatment difference
between the positive control and placebo is above 5 ms. This approach has proven to be useful in many
regulatory cases. However, if the positive control has too large an effect, the study’s ability to detect a 5 ms
QTc prolongation might be questioned.
𝐻0 : ⋂𝑖∈𝑅{𝜇𝑎𝑐𝑡𝑖𝑣𝑒(𝑡𝑖) − 𝜇𝑝𝑙𝑎𝑐𝑒𝑏𝑜(𝑡𝑖) ≤ 5}, versus
𝐻1 : ⋂𝑖∈𝑅{𝜇𝑎𝑐𝑡𝑖𝑣𝑒(𝑡𝑖) − 𝜇𝑝𝑙𝑎𝑐𝑒𝑏𝑜(𝑡𝑖) > 5}.
Where R is a subset of a pre-selected subset of time points; 𝜇𝑎𝑐𝑡𝑖𝑣𝑒(𝑡 ) and 𝜇𝑝𝑙𝑎𝑐𝑒𝑏𝑜(𝑡 ) are mean changes
𝑖
𝑖
from baseline of QT for active drug and placebo respectively, at time point t i.
The authors note that if moxifloxacin is used, then this criterion implicitly requires that the experimental
group respond to moxifloxacin in the same manner as historical control groups. Even if the point estimate
of the difference is only 5 ms, the study will be declared as not having demonstrated assay sensitivity
because moxifloxacin is “known” to produce a certain effect. The authors recommend, based on their
experience with oral moxifloxacin, to reduce risk of failing to establish assay sensitivity by using IV
moxifloxacin to avoid potential issues with other factors such as food effect.
b.
If a positive control has a known effect close to 5 ms, assay sensitivity can be demonstrated if the point
estimate of the maximum mean difference with placebo is close to 5 ms, and the lower bound of the onesided 95% confidence interval for the mean treatment difference between the positive control and placebo
is above 0 ms.
𝐻0 : ⋂𝑖∈𝑅{𝜇𝑎𝑐𝑡𝑖𝑣𝑒(𝑡𝑖) − 𝜇𝑝𝑙𝑎𝑐𝑒𝑏𝑜(𝑡𝑖) ≤ 0}, versus
𝐻1 : ⋂𝑖∈𝑅{𝜇𝑎𝑐𝑡𝑖𝑣𝑒(𝑡𝑖) − 𝜇𝑝𝑙𝑎𝑐𝑒𝑏𝑜(𝑡𝑖) > 0}.
19
Version 1.0
Where R is a subset of a pre-selected subset of time points; 𝜇𝑎𝑐𝑡𝑖𝑣𝑒(𝑡 ) and 𝜇𝑝𝑙𝑎𝑐𝑒𝑏𝑜(𝑡 ) are mean changes
𝑖
𝑖
from baseline of QT for active drug and placebo respectively, at time point t i.
The analyses model of the positive control compared to placebo is similar to the analyses of the test drug compared
to placebo.
7.1.2.1 Multiplicity Issues
Assay sensitivity is usually defined in terms of the statistically significant difference between the positive control
and placebo at one or more post-dose time points.
Due to the multiple comparisons, the probability of demonstrating assay sensitivity is inflated.
To avoid the inflation, the clinical trial sponsor can consider the following options:
Perform the assay sensitivity analysis at fewer post-dose time points. Since the effect of a positive control
on QTc interval is generally well understood, it is reasonable to restrict the positive control versus placebo
comparisons to the number of time points when the QTc effect of the positive control is most pronounced.
For example, if moxifloxacin 400 mg serves as the positive control, significant QT interval prolongation is
likely to occur during the 2-4-hour window after the dose and the sponsor can consider excluding the postdose ECG recordings collected after 10 hours post-dose from the assay sensitivity analysis.
Perform a multiplicity adjustment. When performing this adjustment, it is important to utilize a multiple
testing procedure that takes into account correlations among the estimated treatment differences at postdose time points (e.g., resampling-based multiplicity adjustments, Westfall and Young, 1993). Basic
multiple tests such as the Bonferroni test may be avoided because they tend to be very conservative in
multiplicity problems. The choice of which multiplicity adjustment method to use must be pre-specified
for a specific study.
7.1.3 Categorical Analyses
Categorical (or outlier) analyses are often performed to gain an impression of the proportion of study participants
who exceed predefined upper reference limit values. Outlier reference limits can be defined in terms of absolute
values, change from baseline values or a combination of change from baseline and absolute value. The following
thresholds are often used (but alternative limits may be used):
Absolute QTc interval prolongation:
QTc interval >450 msec
QTc interval >480 msec
QTc interval >500 msec
Change from baseline measurement in QTc interval:
QTc interval increase >30 msec
QTc interval increase >60 msec
20
Version 1.0
It has to be noted that the limits above were selected based on the experience of the writers of this white paper and
ICH E14 guidance. As these limits have their basis in QTcB where QTcF is most commonly used, it is strongly
recommended for the reader to investigate recent literature from the regulators before defining their analysis, as
these recommended limits may change in the future. Change limits should be put in raw numbers or can be
percentage adjusted if empirically derived percentage limits are available.
All outliers should be summarized for each treatment group on at each time point and overall basis. The outlier
summary tables should include counts of subjects (at each time point and overall). Therefore, if a subject
experienced more than one subject of a particular outlier event, the subject should be countered only once for that
event.
Statistical analyses comparing treatments may be performed but is considered out of the scope of this White Paper.
7.1.4 Morphological (Qualitative) Analyses
Morphological (qualitative) abnormal findings (e.g., rhythm; axis; conduction; evidence of ischemia, injury, or
infarction; evidence of hypertrophy; other ST abnormalities; other T-wave abnormalities; U-wave abnormalities;
findings consistent with pericarditis, electrolyte abnormalities, COPD, etc.) in the ECG waveform should be
described and the data presented in terms of the number and percentage of subjects in each treatment arm who had
changes from baseline that represented the appearance or worsening of the morphological abnormality (e.g., tables
of the incidences of the observed treatment emergent abnormalities by specific abnormal finding, not just by
category of findings). Special attention can be directed at abnormalities and/or changes in the appearance of the Twave/U-wave that might be indicative of delayed repolarization, such as double humps ("notched" T wave),
indistinct terminations (TU complex), delayed inscription (prolonged isoelectric ST segment), widening, flattening,
and inversion. T wave alternans (beat-to-beat variability in the amplitude, vector, and/or morphology of the T
wave), is considered to be a harbinger of ventricular arrhythmias and might receive special attention with respect to
occurrence of any of these findings. Several of these T-wave/U-wave findings can be numerically quantified and
analyzed, but this is not a routine expectation in TQT study analyses.
While the predictive value of morphological analyses is not well characterized (even if the drug does have an effect
on the ECG, these abnormal morphological findings will be observed with low frequency if at all in a TQT study),
differences in the incidence of abnormalities between treatment arms, if observed, have proved to be informative.
Statistical analyses comparing treatments may be performed but is considered out of the scope of this White Paper.
7.2 Concentration-Response Relationship (CRR)
Why
a. TQT study is negative (non-inferiority is supported by study results)
When the primary analysis shows evidence of lack of meaningful QT/QTc changes, there still may be small QTc
changes taking place upon administration of the investigational drug at supra-therapeutic doses below the threshold
of regulatory concern. A CRR analysis can clarify whether this is the case or not and inform drug development (e.g.
predict the QTc changes at doses and in subpopulations/factors that were not studied directly). It can also help in
increasing confidence in regards to the timepoints chosen for the primary analysis by investigating possible delayed
effects.
b. TQT study is positive (cannot reject inferiority based on study results)
When the primary analysis does not support lack of QT/QTc prolongation, CRR analysis is an excellent tool to
inform further sponsors and regulators not only about the magnitude of the possible QTc prolongation but also:
help predict the QT effects of doses, dosing regimens, routes of administration, or formulations that were
not studied directly. Interpolation within the range of concentrations studied is considered more reliable
than extrapolation above the range;
21
Version 1.0
inform dose selection for later studies;
inform whether the QTc change occurs simultaneously with the peak concentration (Cmax) or delayed
(e.g., effect-compartment or turnover models);
may assist and clarify the interpretation of equivocal data (on occasion, a TQT study can yield ambiguous
results);
analyses of CRR by sex can be helpful for studying the effect of the drug on QT/QTc interval in cases
where there is evidence or mechanistic theory for a gender difference;
can help predict the effects of intrinsic (e.g., Cytochrome P450 isoenzyme status) or extrinsic (e.g., drugdrug PK interactions) factors, possibly affecting inclusion criteria or dosing adjustments in later phase
studies;
if the results for the study drug are ambiguous (e.g., possible QT prolongation at lower dose but no
prolongation at higher dose or QTc prolongation at a single isolated time point), CRR analysis can help
interpret the data.
When
a. TQT study is negative
If the TQT is negative a PK-QTc analysis is not required by authorities; however when a small drug effect is
expected (based on pre-clinical info, such as hERG test, animal data, etc.) it is a ‘nice to have’.
b. TQT study is positive
As mentioned earlier, the primary IUT analysis is very conservative (the false-positive rate reported in literature
[ICH,2014] is around 20%) and a CRR analysis can either confirm the ICH E14 results as well as provide a nonbiased characterization of the drug effect or point towards further investigation being needed.
c. Assay sensitivity is not demonstrated
CCR might demonstrate that the PK-PD relationship for the positive control is as expected based on historical
control and that failure to demonstrate assay sensitivity was likely due to inadequate positive control exposure due to
one or more of several factors (e.g., delayed absorption of an oral formulation and failure to reach an expected Cmax
due to a food effect when a meal was given shortly before the positive control). Furthermore, it might be possible to
demonstrate that assay sensitivity would have likely been demonstrated if sufficient, and expected, exposure to the
positive control had been achieved.
How
In all situations, it is important that the modeling assumptions, criteria for model selection, and rationale for model
components be specified prior to analysis to limit bias as models with different underlying assumptions on the same
data can produce discordant results. For the same reason pre-specification of model characteristics (e.g., structural
model, objective criteria, goodness of fit) based on knowledge of the pharmacology is recommended whenever
possible.
Mixed effects model can be used to describe the CRR with (Δ)ΔQTc as response (the (Δ)ΔQTc notation will be used
to show that the equation/statement applies both for the ΔQTc and ΔΔQTc subject to the study design). The
following model definition can be considered:
(Δ)ΔQTci (t) = Intercept(i) + drugEffect + eta(i) + eps,
for subject i
where eta(i) stands for subjects i inter-individual variability and eps stands for the residual variability.
The drug effect is given by
(i) in linear effect models
22
Version 1.0
drugEffect = Concentration * Slope
where Slope = drug effect slope
(ii) in power models
drugEffect = Concentrationb
where b = drug effect power
and
(iii) in Emax models
drugEffect = Emax * Concentration / (EC50 + Concentration)
where Emax = maximal effect of the drug on QTc changes
and EC50 = the concentration at which half of the maximal drug effect is reached.
If a time delay is observed between peak concentration and peak QT effect, other models will need to be considered.
These models are considered out-of-scope for this White Paper.
For crossover designs, the ΔΔQTc should be used. For parallel designs, the ΔQTc is used. There are different
opinions for parallel designs whether Placebo observations should be included in the analysis as having zero
concentration; as no formal guidance exists at the time of writing the authors leave it at the readers person
experience but they recommend for the reader to investigate recent literature from the regulators in case such
guidance is issued. The baselines recommended are the same as in the Primary analysis i.e. for crossover designs
“pre-Dose averaged” baseline and for parallel designs “Time-Matched” baseline.
Other considerations for PK/PD
If assay sensitivity is in question based on the results of the primary analysis and PK/PD analysis of the active
control data can be performed to bring confidence in the assay sensitivity claim. The models recommended here are
the same as the ones for the other PK/PD analysis. If Moxifloxacin is to be used then based on Tornøe et al. (2011)
and Florian et al. (2011), we recommend model (i) from the models above.
Finally, the authors stress that a CRR analysis is credible only when the data are well behaved with respect to the
regression line along its entire observed length.
7.3 P-values and Confidence Intervals
There has been an ongoing debate on the value or lack of value for the inclusion of p-values and/or confidence
intervals in safety assessments (Crowe, et. al. 2009). This White Paper does not attempt to resolve this debate. As
noted in the Reviewer Guidance, p-values or confidence intervals can provide some evidence of the strength of the
finding, but unless the trials are designed for hypothesis testing, these should be thought of as descriptive.
Throughout this White Paper, p-values and measures of spread are included in several places. Where these are
included, they should not be considered as hypothesis testing. If a company or compound team decides that these
are not helpful as a tool for reviewing the data, they can be excluded from the display.
Some teams may find p-values and/or confidence intervals useful to facilitate focus, but have concerns that lack of
“statistical significance” provides unwarranted dismissal of a potential signal. Conversely, there are concerns that
due to multiplicity issues, there could be over-interpretation of p-values adding potential concern for too many
outcomes. Similarly, there are concerns that the lower- or upper-bound of confidence intervals will be overinterpreted. A mean change can be as high as x causing undue alarm. It is important for the users of these TFLs to
be educated on these issues if p-values and/or confidence intervals are included in the TFLs.
23
Version 1.0
8 List of outputs
In TQT studies the following list of outputs are commonly produced (for the baseline definitions for Parallel and
Crossover studies, please refer to Section 6.3):
Type
Title
Figure
Individual QT vs. RR plot and QTcF-RR plot
Figure
Box plots of change from baseline in QTc by time-point for
each treatment
Figure
Estimated mean difference in comparison to placebo and
90% CI for change from baseline in QTc (ddQTc) for
treatment
Figure
Estimated mean difference in comparison to placebo and
90% CI for change from baseline in QTc (ddQTc) for
active control
Figure
Mean (+/-SE) change from baseline in QT, QTc and HR by
treatment
Figure
Concentration response for change from baseline in QTc
for active control (assay-sensitivity)
Figure
Mean (+/-SE) QT and QTc intervals by treatment
Figure
Mean (+/-SE) HR by treatment
Figure
Concentration response for change from baseline in QTc
for treatment
Table
Treatment comparisons of change from baseline in QTc
intervals by time for treatment
Table
Treatment comparisons of change from baseline in QTc
intervals by time for active control
Table
Treatment comparisons of change from baseline to all time
points in ECG parameters (HR, PR, QRS) by time for
treatment
Table
Summary of values and changes from baseline to all time
points in ECG parameters by time and treatment
Table
Number and percentage of subjects meeting or exceeding
clinically noteworthy QT and QTc interval changes by time
point and overall
Table
Number and percentage of subjects meeting or exceeding
clinically noteworthy PR, QRS and HR interval changes by
time point and overall
Table
Number and percentage of subjects with abnormal
morphological/qualitative ECG findings
Listing
ECG intervals (average over repeated measurements)
Listing
Change from baseline in ECG intervals(average over
repeated measurements)
Listing
ECG intervals (each replicate)
Listing
ECG interpretation
Listing
ECG findings
24
Version 1.0
9 Outputs shells
Figure 14.2-X.X: Individual QT vs. RR plot and QTcF-RR plot
PROTOCOL/PRODUCT INFO
(page x of x)
Figure 14.2-X.X: Individual QT vs RR plot and QTcF vs RR plot
Analysis set: PD analysis set
Treatment: xxxx
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
25
Version 1.0
Figure 14.2-X.X: Box plots of change from baseline in QTc by time-point for each treatment
PROTOCOL/PRODUCT INFO
(page x of x)
Figure 14.2-X.X: Box plots of change from baseline in QTc by time-point for each treatment
Analysis set: PD analysis set
Cardiac parameter:
XXXXXX
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
26
Version 1.0
Figure 14.2-X.X: Estimated mean difference in comparison to placebo and 90% CI for change from baseline in QTc (ddQTc) for treatment
PROTOCOL/PRODUCT INFO
Figure 14.2-X.X:
(page x of x)
Estimated mean difference in comparison to placebo and 90% CI for change from baseline in QTc (ddQTc) for treatment
Analysis set: PD analysis set
Cardiac parameter: XXXXXX
Treatment: XXXX
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
27
Version 1.0
Figure 14.2-X.X: Estimated mean difference in comparison to placebo and 90% CI for change from baseline in QTc (ddQTc) for active control
PROTOCOL/PRODUCT INFO
Figure 14.2-X.X:
(page x of x)
Estimated mean difference in comparison to placebo and 90% CI for change from baseline in QTc (ddQTc) for active control
Analysis set: PD analysis set
Cardiac parameter: XXXXXX
Treatment: XXXX
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
28
Version 1.0
Figure 14.2-X.X: Mean (+/-SE) change from baseline in QT, QTc, and HR by treatment
PROTOCOL/PRODUCT INFO
Mean (+/-SE) change from baseline in QT, QTc and HR by treatment
(page x of x)
Analysis set: PD analysis set
Cardiac parameter:
Treatment:
time (unit)
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
29
Version 1.0
Figure 14.2-X.X: Mean (+/-SE) QT and QTc intervals by treatment
PROTOCOL/PRODUCT INFO
(page x of x)
Mean (+/-SE) QT and QTc intervals by treatment
Analysis set: PD analysis set
Cardiac parameter:
time (unit)
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
30
Version 1.0
Figure 14.2-X.X: Mean (+/-SE) HR by treatment
PROTOCOL/PRODUCT INFO
Figure 14.2-X.X:
(page x of x)
Mean (+/-SE) HR by treatment
Analysis set: PD analysis set
Cardiac parameter:
time (unit)
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
31
Version 1.0
Figure 14.2-X.X: Concentration-response for change from baseline in QTcF for treatment
PROTOCOL/PRODUCT INFO
Figure 14.2-X.X:
(page x of x)
Concentration response for change from baseline in QTc for treatment
Analysis set: PD analysis set
Compound: XXX, Matrix: YYY, Analyte:ZZZ
Cardiac Parameter:
xxx
Black line is the change from baseline in QTc vs concentration predictions and the grey band is its 90% confidence interval.
Data points depict the raw change from baseline means obtained by grouping at each 10th quantile of concentrations
(each subject contributes only once in the mean, if subject has more than one observation in a quantile the
mean of the subject is obtained prior to the calculation).
Secondary ticks depict the concentration quantiles.
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
32
Version 1.0
Figure 14.2-X.X: Concentration-response for change from baseline in QTc for active control (assay-sensitivity)
PROTOCOL/PRODUCT INFO
(page x of x)
Figure 14.2-X.X: Concentration response for change from baseline in QTc for active control (assay-sensitivity)
Analysis set: PD analysis set
Compound: XXX, Matrix: YYY, Analyte:ZZZ
Cardiac Parameter:
xxx
Black line is the change from baseline in QTc vs concentration predictions and the grey band is its 90% confidence interval.
Data points depict the raw change from baseline means obtained by grouping at each 10th quantile of concentrations
(each subject contributes only once in the mean, if subject has more than one observation in a quantile the
mean of the subject is obtained prior to the calculation).
Secondary ticks depict the concentration quantiles.
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
33
Version 1.0
Table 14.2-X.X: Treatment comparisons of change from baseline at all time points in QTc intervals by time for treatment
PROTOCOL/PRODUCT INFO
Table 14.2-X.X:
(page x of x)
Treatment comparisons of change from baseline at all time points in QTc intervals by time for treatment
Analysis set: PD analysis set
Cardiac parameter:xxxxx
Scheduled time
point (h)
Treatment comparison
x.x
Drug X - Placebo
x.x
x.x
..
x.x
x.x
x.x
Drug X - Placebo
x.x
x.x
..
x.x
Estimate
xx
xx
SE
DF
xxx
90% CI
(xxx, xxx)
p-value*
0.xxx
xx
xx
xxx
(xxx, xxx)
0.xxx
All subjects who have values at both baseline and scheduled time/
relevant visit time period are included in the treatment comparison analysis.
*p-value is the one-sided p-value that Estimate <10
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
34
Version 1.0
Table 14.2-X.X: Treatment comparisons of change from baseline in QTc intervals for active control, by time at all time points
PROTOCOL/PRODUCT INFO
(page x of x)
Table 14.2-X.X: Treatment comparisons of change from baseline to all time points in QTc intervals by time for active control
Analysis set: PD analysis set
Cardiac parameter:xxx
Scheduled time
point(h)
Treatment comparison
x.x
Drug X - Placebo
x.x
x.x
x.x
Estimate
xx
SE
xx
DF
xx
90% CI
(xxx, xxx)
p-value
0.XXX
All subjects who have values at both baseline and scheduled time/
relevant visit time period are included in the treatment comparison analysis.
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
35
Version 1.0
Table 14.2-X.X: Treatment comparisons of change from baseline to all time points in ECG parameters (HR, PR, QRS) by time for treatment
PROTOCOL/PRODUCT INFO
(page x of x)
Table 14.2-X.X: Treatment comparisons of change from baseline to all time points in ECG parameters (HR, PR, QRS) by time for treatment
Analysis set: PD analysis set
Cardiac parameter:xxxxx
Scheduled time
point(h)
x.x
x.x
x.x
..
Treatment comparison
Drug X - Placebo
Estimate
xx
SE
xx
DF
xxx
90% CI
(xxx, xxx)
p-value
0.xxx
All subjects who have values at both baseline and scheduled time/
relevant visit time period are included in the treatment comparison analysis.
PATH DATA/PROGRAM/OUTPUT
36
Version 1.0
Table 14.2-X.X: Summary of values and changes from baseline to all time points in ECG parameters by time and treatment
PROTOCOL/PRODUCT INFO
Table 14.2-X.X: Summary of values and changes from baseline to all time points in ECG parameters
Analysis set: PD analysis set
(page x of x)
by time and treatment
Cardiac parameter:xxxxx
Treatment
N=xxx
Day
XX
Scheduled
time point(h) Statistics
n
x.x
Mean
SD
Min
Q1
Med
Q2
Max
x.x
n
Mean
SD
Min
Q1
Med
Q2
Max
Placebo
N=xxx
p-value
Baseline
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
Post
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
Change
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
Baseline
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
Post
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
Change
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
XXX.XX
Post
Change
0.XXX
0.XXX
0.XXX
0.XXX
- Post = Post Baseline, Change= Post-Baseline - Baseline, Baselie is defined as the time-matched value on Day -1
- Only subjects with values at both baseline and scheduled timepoint are included in the analysis
P-value tests the equivalence of the means of Active vs Placebo at that timepoint
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
37
Version 1.0
Table 14.2-X.X: Number and percentage of subjects meeting or exceeding clinically noteworthy QT and QTc interval limits by time point and overall
PROTOCOL/PRODUCT INFO
(page x of x)
Table 14.2-X.X: Number and percentage of subjects meeting or exceeding clinical noteworthy QT and QTc interval changes by timepoint and overall
Analysis set: PD analysis set
Day
XX
Treatment
N=xx
n/m (%)
Scheduled
time point(h) Variable
x.x
QTcF (ms)
Placebo
N=xx
n/m (%)
p-value*
Increase > 30ms
Increase >60ms
New > 450 ms
New > 480 ms
New > 500 ms
xx/XX
xx/XX
xx/XX
xx/XX
xx/XX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
xx/XX
xx/XX
xx/XX
xx/XX
xx/XX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
0.XXX
0.XXX
0.XXX
0.XXX
0.XXX
Increase > 30ms
Increase >60ms
New > 450 ms
New > 480 ms
New > 500 ms
xx/XX
xx/XX
xx/XX
xx/XX
xx/XX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
xx/XX
xx/XX
xx/XX
xx/XX
xx/XX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
0.XXX
0.XXX
0.XXX
0.XXX
0.XXX
QT (ms)
*
n : Number of subjects who meet the designated criterion
m: Number of subjects at risk for a designated change with a non missing value at baseline and postbaseline
N: Total number of subjects in the treatment group in this analysis set
P-value compares the probability of the clinicaly noteworthy event of active vs Placebo
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
38
Version 1.0
Table 14.2-X.X: Number and percentage of subjects meeting or exceeding clinically noteworthy PR, QRS and HR interval limits by time point and overall
PROTOCOL/PRODUCT INFO
(page x of x)
Table 14.2-X.X: Number and percentage of subjects meeting or exceeding clinically noteworthy PR, QRS and HR interval changes by timepoint and overall
Analysis set: PD analysis set
Day: X
Scheduled
time point(h) Variable
x.x
PR increase > 25% to a value > 200 ms
QRS increase > 25% to a value > 120 ms
HR decrease > 25% to a HR < 50 bpm
HR increase > 25% to a HR > 100 bpm
x.x
PR increase > 25% to a value > 200 ms
QRS increase > 25% to a value > 120 ms
HR decrease > 25% to a HR < 50 bpm
HR increase > 25% to a HR > 100 bpm
Treatment
N=xx
n/m (%)
Placebo
N=xx
n/m (%)
p-value*
xx/XX
xx/XX
xx/XX
xx/XX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
xx/XX
xx/XX
xx/XX
xx/XX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
0.XXX
0.XXX
0.XXX
0.XXX
xx/XX
xx/XX
xx/XX
xx/XX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
xx/XX
xx/XX
xx/XX
xx/XX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
0.XXX
0.XXX
0.XXX
0.XXX
- n : Number of subjects who meet the designated criterion
- m: Number of subjects at risk for a designated change with a non missing value at baseline and postbaseline
- N: Total number of subjects in the treatment group in this analysis set
'* P-value compares the probability of the clinicaly noteworthy event of active vs Placebo
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
39
Version 1.0
Table 14.2-X.X: Number and percentage of subjects with abnormal morphological/qualitative ECG findings
PROTOCOL/PRODUCT INFO
Table 14.2-X.X: Number and percentage of subjects with abnormal morphological/qualitative ECG findings
Analysis set: PD analysis set
(page x of x)
Day: X
Treatment
Abnormality Type Finding
Any ECG abnormality
Rhythm
Atrial Flutter
Atrial Fibrillation
Junctional Rhythm
…
Morphology
RAA
LAA
…
Placebo
Baseline
N= xx
n(%)
xx (xx.x)
Postbaseline
N= xx
n(%)
xx (xx.x)
Postbaseline
Baseline
N= xx
N= xx
n(%)
n(%)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
0.XXX
0.XXX
0.XXX
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
xx (xx.x)
0.XXX
0.XXX
xx
xx
xx
xx
xx
xx
xx
xx
xx
xx
xx
xx
xx
xx
xx
xx
0.XXX
0.XXX
0.XXX
0.XXX
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
(xx.x)
p-value
0.XXX
N is the number of subjects with a valid pre-dose and with at least one observation post dose and is used as the denominator
in the calculation of the percentages.
“n” for baseline is the number of subjects with an ECG abnormality at least at one time point at baseline
“n” for post-baseline is the number of subjects with an ECG abnormality at least at one post dose value for the Treatment columns.
“new” is the number of subjects who have an abnormal ECG finding at post dose which is not present at pre-dose.
A subject with multiple occurrences of an abnormality is counted only once for the corresponding treatment or for pre-dose.
* P-value compares the probability of the post-dose abnormality event of active vs Placebo
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
40
Version 1.0
Listing 16.2.9-X.X: ECG intervals (average over repeated measurements)
PROTOCOL/PRODUCT INFO
Listing 16.2.9-X.X: ECG intervals (average over repeated measurements)
Analysis set: PD analysis set
(page x of x)
Treatment / Treatment sequence: xxxx
Country/
Site/
Subject
Age/
Sex/ Visit/
Race Day
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
Scheduled
time
point (h)
QT
(ms)
QTcI
(ms)
QTcF
(ms)
QTcB
(ms)
PR
(ms)
QRS
(ms)
RR
(ms)
HR
(bpm)
x.x
x.x
x.x
xx
xx
xx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
x
x
x
xx
xx
xx
x
x
x
x.x
x.x
x.x
xx
xx
xx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
x
x
x
xx
xx
xx
x
x
x
+ : QT or QTc > 450 ; ++ : QT or QTc >480 ; +++ QT or QTc > 500
* : HR <50 ; ** : HR > 100
^ : PR > 200
@ : QRS > 120
& : RR < 600 ; &&: RR > 1200
! : Value has been excluded from the PD analysis
ECG interval values are based on the average of the repeated measurements within the same scheduled time point.
Flags are applied to the average of the repeated measurements.
Scheduled time includes both baseline and post baseline time points.
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
41
Version 1.0
Listing 16.2.9-X.X: Change from baseline in ECG intervals (average over repeated measurements)
PROTOCOL/PRODUCT INFO
(page x of x)
Listing 16.2.9-X.X: Change from baseline in ECG intervals (average over repeated measurements
Analysis set: PD analysis set
Treatment / Treatment sequence: xxxx
Country/ Age/
Site/
Sex/ Visit/
Subject Race Day
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
Scheduled
time
point (h)
QT
(ms)
QTcI
(ms)
QTcF
(ms)
QTcB
(ms)
PR
(ms)
QRS
(ms)
RR
(ms)
HR
(bpm)
x.x
x.x
x.x
xx
xx
xx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
x
x
x
xx
xx
xx
x
x
x
x.x
x.x
x.x
xx
xx
xx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
x
x
x
xx
xx
xx
x
x
x
ECG interval values are based on the average of the repeated measurements within the same scheduled
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
42
Version 1.0
Listing 16.2.9-X.X: ECG intervals (each replicate)
PROTOCOL/PRODUCT INFO
Listing 16.2.9-X.X: ECG intervals (each replicate)
(page x of x)
Analysis set: PD analysis set
Treatment / Treatment sequence: xxxx
Country Age/
/ Site/ Sex/ Visit/
Subject Race Day
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
+
*
^
@
&
!
:
:
:
:
:
:
Scheduled
time
ECG time
point (h) (hh:mm:ss)
QT
(ms)
QTcI
(ms)
QTcF
(ms)
QTcB
(ms)
PR
(ms)
QRS
(ms)
RR
(ms)
HR
(bpm)
x.x
x.x
x.x
hh:mm:ss
hh:mm:ss
hh:mm:ss
xx
xx
xx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
x
x
x
xx
xx
xx
x
x
x
x.x
x.x
x.x
hh:mm:ss
hh:mm:ss
hh:mm:ss
xx
xx
xx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
x
x
x
xx
xx
xx
x
x
x
QT or QTc > 450 ; ++ : QT or QTc >480 ; +++ QT or QTc > 500
HR <50 ; ** : HR > 100
PR > 200
QRS > 120
RR < 600 ; &&: RR > 1200
Value has been excluded from the PD analysis
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
43
Version 1.0
Listing 16.2.9-X.X: T-wave and U-wave morphology
PROTOCOL/PRODUCT INFO
Listing 16.2.9-X.X: T-wave and U-wave morphology
Analysis set: PD analysis set
(page x of x)
Treatment / Treatment sequence: xxxx
Country/
Site/
Subject
Age/
Sex/ Visit/
Race Day
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
Scheduled
time point
(h)
ECG Time
(hh:mm:ss)
T-wave
morphology
ST
Segment
U-wave
present
x.x
x.x
x.x
hh:mm:ss
hh:mm:ss
hh:mm:ss
xxxxx
xxxxx
xxxxx
xxxx
xxxx
xxxx
xxxxx
xxxxx
xxxxx
x.x
x.x
x.x
hh:mm:ss
hh:mm:ss
hh:mm:ss
xxxxx
xxxxx
xxxxx
xxxx
xxxx
xxxx
xxxxx
xxxxx
xxxxx
! : Value has been excluded from the PD analysis
ST segment: all terms
T waves: all terms
U waves: all terms
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
44
Version 1.0
Listing 16.2.9-X.X: ECG findings
PROTOCOL/PRODUCT INFO
(page x of x)
Listing 16.2.9-X.X: ECG findings
Analysis set: PD analysis set
Treatment / Treatment sequence: xxxx
Country/
Site/
Subject
Age/
Sex/ Visit/
Race Day
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
CNTR /
ST1/
XXXXX
YY/
M/
Ca
x/
ddMMyy
ECG Interpretation
Comments
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
xxxxx
PATH DATA/PROGRAM/OUTPUT
PRODUCTION STATUS/RUN DDMMYYYY: HHMM
45
Version 1.0
10 References
(ICH), International Conference on Harmonisation. "Guidance for Industry E14 Clinical Evaluation of QT/QTc
Interval Prolongation and Proarrhythmic Potential for Non-antiarrhythmic drugs Questions and Answers
R1." n.d.
http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073161.
pdf (accessed July 25, 2014).
—. "Guidance for Industry E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential
for Non-antiarrhythmic drugs Questions and Answers R2." n.d.
Berger, R. L., Hsu, J. C. "Bioequivalence trials, intersection-union tests and equivalence confidence sets." Statistical
Science, 1996: 11, 283-319.
Bonnemeier, H., Wiegand, U. K., Braasch, W., Brandes, A., Richardt, G., POTRATZ, J. "Circadian profile of QT
interval and QT interval variability in 172 healthy volunteers." Pacing and clinical electrophysiology,
2003: 26(1p2), 377-382.
Couderc, J. P., Xiaojuan, X., Zareba, W., Moss, A. J. "Assessment of the Stability of the Individual‐Based
Correction of QT Interval for Heart Rate." Annals of Noninvasive Electrocardiology, 2005: 10, 25-34.
F, Dessertenne. "Ventricular tachycardia with 2 variable opposing foci (translation of theFrench)." Arch Coeur
Vaiss, 1966: 59:263-272.
Florian, A.F., Tornøe, C.W., Brundage, R., Parekh, A., Garnett, C.E. "Population Pharmacokinetic and
Concentration-QTc Models for Moxifloxacin: Pooled Analysis of 20 Thorough QT Studies." Journal of
Clinical Pharmacology, 2011: 51, 1152-1162.
Garnett, C. E., Beasley, N., Bhattaram, V. A., Jadhav, P. R., Madabushi, R., Stockbridge, N., Gobburu, J. V.
"Concentration‐QT Relationships Play a Key Role in the Evaluation of Proarrhythmic Risk During
Regulatory Review." The Journal of Clinical Pharmacology, 2008: 48, 13-18.
Garnett, C. E., Zhu, H., Malik, M., Fossa, A. A., Zhang, J., Badilini, F., Rodriguez, I. "Methodologies to
characterize the QT/corrected QT interval in the presence of drug-induced heart rate changes or other
autonomic effects. " American Heart Journal, 2012: 163, 912-930.
Jervell, A., Lange-Nielsen, F. " Congenital deaf-mutism, functional heart disease with prolongation of the QT
interval, and sudden death. ." American heart journal, 1957: 54, 59-68.
Karlsson, M. O., & Sheiner, L. B. "The importance of modeling interoccasion variability in population
pharmacokinetic analyses." Journal of pharmacokinetics and biopharmaceutics, 1993: 21, 735-750.
Malik, M. " Problems of Heart Rate Correction in Assessment of Drug‐Induced QT Interval Prolongation. ." Journal
of cardiovascular electrophysiology, 2001: 12, 411-420.
Malik, M. "The imprecision in heart rate correction may lead to artificial observations of drug induced QT interval
changes." Pacing and clinical electrophysiology, 2002: 25, 209-216.
Malik, M., Andreas, J. O., Hnatkova, K., Hoeckendorff, J., Cawello, W., Middle, M., Braun, M. "Thorough QT/QTc
study in patients with advanced Parkinson's disease: cardiac safety of rotigotine." Clinical Pharmacology &
Therapeutics, 2008: 84, 595-603.
Malik, M., Färbom, P., Batchvarov, V., Hnatkova, K., & Camm, A. J. "Relation between QT and RR intervals is
highly individual among healthy subjects: implications for heart rate correction of the QT interval." Heart,
2002: 87, 220-228.
Malik, M., Hnatkova, K., Batchvarov, V., Gang, Y., Smetana, P., CAMM, A. "Sample size, power calculations, and
their implications for the cost of thorough studies of drug induced QT interval prolongation." Pacing and
clinical electrophysiology, 2004: 27, 1659-1669.
Malik, M., Hnatkova, K., Novotny, T., Schmidt, G. "Subject-specific profiles of QT/RR hysteresis." American
Journal of Physiology-Heart and Circulatory Physiology, 2008: 295, H2356-H2363.
Meng, Z., Kringle, R., Chen, X., & Zhao, P. L. "Sample size calculation for thorough QT/QTc study considering
various factors related to multiple time points." Journal of biopharmaceutical statistics, 2010: 20, 580-594.
Morganroth, J. "Design and conduct of the Thorough Phase I ECG trial for new bioactive drugs. In Cardiac Safety
of Noncardiac Drugs." Humana Press, 2005: 205-222.
Patterson S, Agin M, Anziano R, Burgess T, Chuang-Stein C, Dmitrienko A, Ferber G,Geraldes M, Ghosh K,
Menton R, Natarajan J, Offen W, Saoud J, Smith B, Suresh R, Zariffa N. "Investigating drug-induced QT
and QTc prolongation in the clinic: A review of statistical design and analysis considerations. Report from
the Pharmaceutical Research and Manufacturers of America QT Statistics Expert Team." Drug Information
Journal., 2005: 39, 243-266.
46
Version 1.0
Piotrovsky, V. "Pharmacokinetic-pharmacodynamic modeling in the data analysis and interpretation of druginduced QT/QTc prolongation." The AAPS Journal, 2005: 7, E609-E624.
Sethuraman, V., & Sun, Q. "Impact of baseline ECG collection on the planning, analysis and interpretation of
‘thorough’QT trials." Pharmaceutical Statistics, 2009: 8, 113-124.
Smirk FH, Palmer DG. "A myocardial syndrome: with particular reference to the occurance of sudden death and of
premature systoles interrupting antecedent T waves." American Journal of Cardiology, 1960: 6:620-629.
Sun, G., Quan, H., Kringle, R., Meng, Z. "Comparison of statistical models adjusting for baseline in the analysis of
parallel-group thorough QT/QTc studies. ." Journal of biopharmaceutical statistics, 2012: 22, 438-462.
Tornøe, C.W., Garnett, C.E., Wang, Y., Florian, J., Li, M., Gobburu, J.V. "Creation of a Knowledge Management
System for QT Analyses." The Journal of Clinical Pharmacology, 2011: 51, 1035-1042.
Westfall PH, Young SS. Resampling-based multiple testing: examples and methods for p-value adjustment. Wiley,
1993.
47