* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CO 2 (g)
Survey
Document related concepts
X-ray fluorescence wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Transition state theory wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Energy harvesting wikipedia , lookup
Thermodynamics wikipedia , lookup
Transcript
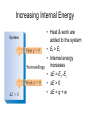
Thermochemistry Chapter 5 Introduction • All chemical and physical changes involve energy • Thermodynamics is the study of energy and its transformations • Thermochemistry is the study of the energy changes involved in chemical reactions 5.1 The Nature of Energy Key Concepts • • • • • • • • • Thermodynamics Thermochemistry Kinetic energy Potential energy System Surroundings Work Force Heat 5.1 The Nature of Energy • • • • Three fundamental forms of energy Kinetic energy Potential energy Mass energy E = mc2 discussed elsewhere Kinetic Energy • • • • • • • • • Any object in motion possesses KE or Ek KE = ½ mv2 Examples A cow of mass 2000 kg, walking at a speed of 0.5 m/s has…. KE = 0.5 x 2000 kg x (0.5 m/s)2 = 250 Joules KE 1 J = 1 kg∙m2/s2 How fast would a 2.0 g fly have to fly to have the same KE? = 500 m/s =1118 mph Potential Energy • Potential energy is “stored” energy • Energy possessed by an object by virtue of its position relative to other objects • Gravity imparts PE PE = mgh • Electrostatic and chemical energy are forms of PE Energy Content • Total energy of an object is the sum of KE and all forms of PE • E tot = KE + Σ PE • Example: A biker at the top of a hill has PE relative to the bottom of the hill A biker riding down the hill has both KE & PE As a biker reaches the bottom of the hill, all PE has been converted to KE Conservation of Energy Conservation of Mechanical Energy 0.80 Energy (J) 0.70 0.60 PE 0.50 KE 0.40 ME 0.30 Poly. (PE) 0.20 Poly. (KE) 0.10 Linear (ME) 0.00 -0.100.00 0.10 0.20 0.30 Time (s) 0.40 0.50 Units of Energy • Joule 1 J = 1 N∙m = 1kg∙m2/s2 • 1 cal = 4.184 J • calorie The energy required to increase the temperature of 1 g of water by 1°C • Nutritional calories … • Are actually kilocalories • 1 Cal = 1000 cal = 1 kcal System & Surroundings • Thermodynamic studies are conducted within a defined system • System: portion of the universe defined for study • Surroundings: everything else • Open system can exchange energy with surroundings • Close system cannot exchange energy and matter with surroundings self-contained – self-contained – Isolated from surroundings Transferring Energy: Work and Heat • What is work? • Force: a push or pull exerted on an object • Work is the energy used to cause an object to move • Work is the product of Force x distance • w = Fd Units? • Energy is transferred as work Heat • Energy is also transferred as heat. • What is heat? • Heat is energy transferred from an object of higher temperature to an object of lower temperature • Heat is not temperature! Energy • So energy can be defined as… • Capacity to do work or to transfer heat • ΔE = w + q Sample Problem • a) b) c) d) A bowler lifts a 5.4-kg bowling ball from ground level to a height of 1.6 m, and then drops it back to the ground What happens to PE as it is raised? How much work was done to raise the ball? What is the speed of the ball at impact? What is the weight of the ball in newtons? More sample problems • Calculate the KE in J for • An Ar atom moving with a speed of 650 m/s • A mole of Ar atoms moving at the speed of 650 m/s 5.2 Key Concepts • • • • • Internal energy First law of thermodynamics Endothermic Exothermic State functions 5.2 The First Law of Thermodynamics • Energy is conserved • Energy is neither created nor destroyed, but can be transformed. • The total energy of the universe is a constant First Law of Thermodnamics •If the energy of a system decreases, it must be transferred to the surroundings •If energy is gained by a system, it must be transferred from the surroundings Internal Energy • Sum of all KE and PE of all components of a system • E = ΣKE + ΣPE • Change in internal energy is … • ∆E = Ef - Ei • E cannot be known, but ∆E can be measured Internal Energy E = the sum of all kinetic and potential energies of all components of the system Internal Energy • Thermodynamic quantities must be described with a number, a unit, and a sign • When the system has absorbed energy from its surroundings… • +∆E….. Ef > Ei, therefore ∆E is positive • When the system loses energy to its surroundings… • -∆E ….. Ef < Ei, therefore ∆E is negative Changing Internal Energy • A system of H2 and O2 gas has more internal energy than one composed of H2O (l) • E is lost to surroundings when H2O (l)is formed • E is gained when H2O (l) is decomposed Increasing Internal Energy • Heat & work are added to the system • Ef > Ei • Internal energy increases • ∆E = Ef -Ei • ∆E > 0 • ∆E = q + w Sample Problem Relating ∆E to heat & work • H2(g) & O2(g) ignite • System loses 1150J of heat to surroundings • As gases expand, piston rises • 480 J work done on the piston. • What ∆E? • ∆E = q + w • ∆E = -1150J + (-)480J • ∆E = -1630 J • 1630 J was transferred from system to the surroundings Endothermic & Exothermic Processes • Endothermic…. • System absorbs heat from surroundings • Exothermic…. • System releases heat into surroundings Energy Diagram Exothermic Reaction State – specific characteristics of a system (physical or chemical) Examples: Pressure Temperature Composition State Function – a property of a system that characterizes a state of a system and is independent of how the system got to that state. Examples: Pressure Volume Composition www70.homepage.villanova.edu/kathleen.thrush/chapter_6_powerpoint_le.ppt Thermodynamics State functions are properties that are determined by the state of the system, regardless of how that condition was achieved. energy, pressure, volume, temperature DE = Efinal - Einitial DP = Pfinal - Pinitial DV = Vfinal - Vinitial DT = Tfinal - Tinitial Potential energy of hiker 1 and hiker 2 is the same even though they took different paths. Energy it took each hiker to get to the top is different !! www70.homepage.villanova.edu/kathleen.thrush/chapter_6_powerpoint_le.ppt State Functions • In the first law of thermodynamics ΔE = q + w • ΔE is a state function • q and w are not • See fig 5.8 p. 152 5.3 Enthalpy • Symbol H • Like internal energy, enthalpy cannot be measured, but ∆H can • ∆H equals the heat, qp, gained or lost by a system under constant pressure • Exothermic processes: ∆H < 0 • Endothermic processes: ∆H > 0 • ∆H = Hfinal – Hinitial = qp 5.4 Enthalpies of Reaction Thermochemical Equations • ∆H = Hfinal – H initial • ∆Hrxn = Hproducts – Hreactants • 2H2 (g) + O2 (g) 2H2O (g) ∆H = -486.3 kJ • When 2 moles of H2 gas combusts to form 2 moles of H2O under conditions of constant pressure, 486.3 kJ of heat are released by the system. • Coefficients indicate molar amounts associated with ∆Hrxn Enthalpy Diagrams Guidelines for Thermochemical Equations 1. Enthalpy is an extensive property CH4(g) + O2(g) CO2(g) + 2H2O(l) ∆H = -890 kJ 2. ∆H for reaction is equal in magnitude but opposite in sign to ∆H for the reverse rxn CO2(g) + 2H2O(l) CH4(g) + O2(g) ∆H = +890 kJ 3. ∆H depends on the state of the reactants & products CH4(g) + O2(g) CO2(g) + 2H2O(g) ∆H = -802 kJ 2H2O(l) 2H2O(g) ∆H = +88 kJ 5.5 Calorimetry • Calorimetry is the measure of heat flow involved in a chemical reaction • Heat capacity: amount of heat energy required to raise the temperature of an object by 1K • Molar heat capacity: amount of heat energy required to raise the temperature of 1 mole of a pure substance by 1K • Specific heat: amount of heat energy required to raise the temperature of 1 g of a substance by 1K • = heat capacity of 1 gram Determining specific heat • During a thermochemical process, – Measure mass of substance – Measure change in temperature – Measure heat energy transferred Specific heat = q/mΔT Units: J/g∙K q = mc(Tf-Ti) Sample Problem 5.5 • How much heat is needed to warm 250g of water from 22C to 98C. (Specific heat of water is 4.18 J/gK) • Determine the molar heat capacity of water Constant Pressure Calorimetry • Calorimeter • An insulated container in which aqueous chemical reactions are conducted • If the mass and specific heat of the solvent (water) is known, q can be calculated if ΔT of the water is measured • qsoln = - qrxn and qrxn = - qsoln • qrxn = - (msoln)(csoln )ΔT Sample 5.6 • 50 mL of 1.0 M HCl mixed with 50 mL of 1.0 M NaOH. • Given: Ti = 21.0C Tf = 27.5C; density of soln = 1.0 g/mL; csoln = 4.18 J/gK • Calculate enthalpy change for the neutralization reaction • msoln = V x d = 100mL x 1.0 g/mL = 100 g • ΔTsoln = Tf - Ti = 27.5 – 21.0C = 6.5C • = 6.5K Sample 5.6 • qrxn = - qsoln • = - (msoln)(csoln )ΔTsoln • = - (100 g)(4.18J/gK)(6.5K) • = - 2700 J • = - 2.7 kJ • How would you express this change on a molar basis? Sample 5.6 • • • • At constant pressure ΔH = q Express ΔH on a molar basis HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) Molar quantities of reacants = (0.050 L)(1 mol/L) = 0.050 mol • ΔH = (-2.7 kJ/0.050 mol) = -54 kJ/mol 5.6 Hess’s Law • If a rxn is carried out in steps, ΔHrxn = sum of ΔH of each step. • ΔHrxn = Σ ΔH1 + ΔH2 + ΔH3 + …step. AD ΔHrxn = -850 kJ net rxn AB ΔH1 = -175 kJ step 1 BC ΔH2 = -400 kJ step 2 C D ΔH3 = -275kJ step 3 Hess’s Law Eq 1 CH4(g) + 2O2(g) CO2(g) + 2H2O(g) ΔH = -802 kJ Eq 2 2H2O (g) 2H2O (l) ΔH = -88 kJ Add Eq 1 + Eq 2 CH4(g)+2O2(g)+2H2O (g) CO2(g)+2H2O(g)+2H2O (l) ΔH = -890 kJ Net Eq CH4(g) + 2O2(g) CO2(g) + 2H2O (l) ΔH = -890 kJ Using Hess’s Law to Calculate ΔH Sample 5.8 Given: (1) C(s) + O2(g) → CO2(g) (2) CO(g) + ½ O2(g) → CO2(g) ΔH1 = -393.5 kJ ΔH2 = -283.0 kJ Determine the ΔH for the reaction (3) C(s) + ½ O2 (g) → CO(g) ΔH3 = ? kJ Using Hess’s Law to Calculate ΔH Sample 5.8 The basic idea: rearrange equations 1 and 2, if necessary, in such a way that, when added, produce equation 3 C(s ) O 2 ( g ) CO 2 ( g ) DH1 - 393.5 kJ 1 CO 2 ( g ) CO(g ) O 2 ( g ) - DH 2 283.0 kJ 2 _____________________________________________ 1 C(s ) O 2 ( g ) CO(g ) DH 3 - 110.5 kJ 2 5.7 Enthalpies of Formation • Thermodynamic quantities: • ΔHº Standard Enthalpy Standard conditions: P = 1 atm, T = 298K • ΔHvap Enthalpy of vaporization Vaporize liquids to gases • ΔHf Enthalpy of fusion Melting solids to liquids • ΔHc • ΔHfº Enthalpy of combustion Enthalpy of formation Enthalpy of Formation • ΔHfº for the formation of 1 mol of a substance from its elements • 2 C (graphite) + 3H2 (g) + ½ O2 (g) C2H5OH (l) • ΔHfº = -277.7 kJ • By definition, ΔHfº = 0 for any element Calculation of DH using DHf° We can use Hess’s law in this way: DH = nDHf°products – mDHf° reactants where n and m are the stoichiometric coefficients. Calculation of DH using DHf° C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) DH = [3(-393.5 kJ) + 4(-285.8 kJ)] – [1(-103.85 kJ) + 5(0 kJ)] = [(-1180.5 kJ) + (-1143.2 kJ)] – [(-103.85 kJ) + (0 kJ)] = (-2323.7 kJ) – (-103.85 kJ) = -2219.9 kJ Energy in Foods Most of the fuel in the food we eat comes from carbohydrates and fats. Energy in Fuels The vast majority of the energy consumed in this country comes from fossil fuels.