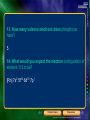
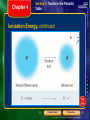
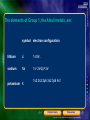
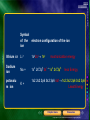
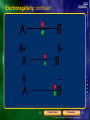
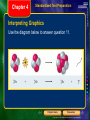
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 4 - My Chemistry Site
Survey
Document related concepts
Transcript
How to Use This Presentation • To View the presentation as a slideshow with effects select “View” on the menu bar and click on “Slide Show.” • To advance through the presentation, click the right-arrow key or the space bar. • From the resources slide, click on any resource to see a presentation for that resource. • From the Chapter menu screen click on any lesson to go directly to that lesson’s presentation. • You may exit the slide show at any time by pressing the Esc key. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Resources Chapter Presentation Bellringer Transparencies Standardized Test Prep Visual Concepts Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 The Periodic Table Table of Contents Section 1 How Are Elements Organized? Section 2 Tour of the Periodic Table Section 3 Trends in the Periodic Table Section 4 Where Did the Elements Come From? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 1 How Are Elements Organized? Bellringer • Make a list of things in the classroom that you think are made from single elements. Make sure you think about things you cannot see such as air. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 1 How Are Elements Organized? Objectives • Describe the historical development of the periodic table. • Describe the organization of modern periodic table according to the periodic law. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 1 How Are Elements Organized? The Periodic Law • Mendeleev’s principle of chemical periodicity is known as the periodic law, which states that when the elements are arranged according to their atomic numbers, elements with similar properties appear at regular intervals. Organization of the Periodic Table • Elements in each column of the periodic table have the same number of electrons in their outer energy level. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 1 How Are Elements Organized? The Periodic Law, continued Organization of the Periodic Table, continued • The electrons in the outer shell are called valence electrons. • Valence electrons are found in the outermost shell of an atom and that determines the atom’s chemical properties. • Elements with the same number of valence electrons tend to react in similar ways. • Because s and p electrons fill sequentially, the number of valence electrons in s- and p-block elements are predictable. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 1 How Are Elements Organized? Blocks of the Periodic Table Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 1 How Are Elements Organized? The Periodic Law, continued Organization of the Periodic Table, continued • A vertical column on the periodic table is called a group. Elements in a group share chemical properties. • A horizontal row on the periodic table is called a period. Elements in the same period have the same number of occupied energy levels. • Example: all elements in Period 2 have atoms whose electrons occupy two principal energy levels, including the 2s and 2p orbitals. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 1 How Are Elements Organized? The Periodic Law, continued Organization of the Periodic Table, continued • The periodic table provides information about each element. • atomic number • symbol • name • average atomic mass • electron configuration Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Periodic Table Overview Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. HOMEWORK SECTION REVIEW Pg. 122 Q (5-14) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Section 4.1 Review, pg.122 5. State the periodic law. When elements are arranged according to their atomic numbers, elements with similar properties appear at regular intervals. 6. What do elements in the same period have in common? They have the same number of occupied energy levels 7. What do elements in the same group have in common? The same number of valence electrons Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 8. Why can Period 1 contain a maximum of two elements? The first energy level of an atom can contain only two electrons. 9. In which period and group is the element whose electron configuration is [Kr]5s1? Period 5, group 1 10. Write the outer electron configuration for the Group 2 element in Period 6. 6S2 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 11. What determines the number of elements found in each period in the periodic table? The number of s and p electrons in that level + previous level d- electrons + next previous level of f- electrons 12. Are elements with similar chemical properties more likely to be found in the same period or in the same group? Explain your answer Same group, because they have same number of valence electrons. Valence electrons determine the chemical properties of the elements. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 13. How many valence electrons does phosphorus have? 5 14. What would you expect the electron configuration of element 113 to be? [Rn] 7s2 5f14 6d10 7p1 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Bellringer • On a blank periodic table, label each group by the electron configuration of the valence electrons, assuming the configuration follows the pattern given by the aufbau principle. • This pattern applies to all the main-group elements, but there are many exceptions in the transition metals in the center of the table. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Objectives • Locate the different families of main-group elements on the periodic table, describe their characteristic properties, and relate their properties to their electron configuration. • Locate metals on periodic table, describe their characteristic properties, and relate their properties to their electron configuration. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements • Elements in groups 1, 2, and 13–18 are known as the main-group elements. Main-group elements are in the s- and p-blocks of the periodic table. • The electron configurations of the elements in each main group are regular and consistent: the elements in each group have the same number of valence electrons. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued • The main-group elements are sometimes called the representative elements because they have a wide range of properties. • At room temperature and atmospheric pressure, many are solids, while others are liquids or gases. • About half of the main-group elements are metals. • Many are extremely reactive, while several are nonreactive. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued • The main-group elements silicon and oxygen account for four of every five atoms found on or near Earth’s surface. • Four groups within the main-group elements have special names. These groups are: • alkali metals (Group 1) • alkaline-earth metals (Group 2) • halogens (Group 17) • noble gases (Group 18) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued • Main-group are highlighted in the groups on the left and right sides of the periodic table. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued The Alkali Metals Make Up Group 1 • Elements in Group 1 are called alkali metals. • lithium, sodium, potassium, rubidium, cesium, and francium • Alkali metals are so named because they are metals that react with water to make alkaline solutions. • Because the alkali metals have a single valence electron, they are very reactive. • In losing its one valence electron, potassium achieves a stable electron configuration. • Alkali metals are never found in nature as pure elements but are found as compounds. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued The Alkali Metals Make Up Group 1, continued Physical Properties of Alkali Metals Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued The Alkaline-Earth Metals Make Up Group 2 • Group 2 elements are called alkaline-earth metals. • The alkaline-earth metals are slightly less reactive than the alkali metals. • They are usually found as compounds. • The alkaline-earth metals have two valence electrons and must lose both their valence electrons to get to a stable electron configuration. • It takes more energy to lose two electrons than it takes to lose just the one electron that the alkali metals must give up to become stable. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued The Halogens, Group 17, Are Highly Reactive • Elements in Group 17 of the periodic table are called the halogens. • The halogens are the most reactive group of nonmetal elements. • When halogens react, they often gain the one electron needed to have eight valence electrons, a filled outer energy level. • Because the alkali metals have one valence electron, they are ideally suited to react with the halogens. • The halogens react with most metals to produce salts. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued The Noble Gases, Group 18, Are Unreactive • Group 18 elements are called the noble gases. • The noble gas atoms have a full set of electrons in their outermost energy level. • The low reactivity of noble gases leads to some special uses. • The noble gases were once called inert gases because they were thought to be completely unreactive. • In 1962, chemists were able to get xenon to react, making the compound XePtF6. • In 1979, chemists were able to form the first xenon-carbon bonds. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table The Main-Group Elements, continued Hydrogen Is in a Class by Itself • Hydrogen is the most common element in the universe. • It is estimated that about three out of every four atoms in the universe are hydrogen. • Because it consists of just one proton and one electron, hydrogen behaves unlike any other element. • Hydrogen is in a class by itself in the periodic table. • With its one electron, hydrogen can react with many other elements, including oxygen. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals • The majority of elements, including many main-group ones, are metals. • Metals are recognized by its shiny appearance, but some nonmetal elements, plastics, and minerals are also shiny. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals, continued The regions highlighted in blue indicate the elements that are metals. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals, continued Metals Share Many Properties • All metals are excellent conductors of electricity. • Electrical conductivity is the one property that distinguishes metals from the nonmetal elements. • Some metals, such as manganese, are brittle. • Other metals, such as gold and copper, are ductile and malleable. • Ductile means that the metal can be squeezed out into a wire. • Malleable means that the metal can be hammered or rolled into sheets. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Properties of Metals: Malleability and Ductility Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals, continued Transition Metals Occupy the Center of the Periodic Table • The transition metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. • A transition metal is one of the metals that can use the inner shell before using the outer shell to bond. • A transition metal may lose one, two, or even three valence electrons depending on the element with which it reacts. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals, continued Transition Metals Occupy the Center of the Periodic Table, continued • Generally, the transition metals are less reactive than the alkali metals and the alkaline-earth metals are. • Some transition metals are so unreactive that they seldom form compounds with other elements. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals, continued Lanthanides and Actinides Fill f-orbitals • The elements in the first of these rows are called the lanthanides because their atomic numbers follow the element lanthanum. • A lanthanide is a member of the rare-earth series of elements, whose atomic numbers range from 58 (cerium) to 71 (lutetium). • Elements in the row below the lanthanides are called actinides because they follow actinium. • An actinide is any of the elements of the actinide series, which have atomic numbers from 89 (actinium, Ac) through 103 (lawrencium, Lr). Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals, continued Lanthanides and Actinides Fill f-orbitals, continued • As one moves left to right along these rows, electrons are added to the 4f orbitals in the lanthanides and to the 5f orbitals in the actinides. • The lanthanides and actinides are sometimes called the f-block of the periodic table. • The actinides are unique in that their nuclear structures are more important than their electron configurations. • Because the nuclei of actinides are unstable and spontaneously break apart, all actinides are radioactive. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 2 Tour of the Periodic Table Most Elements Are Metals, continued Other Properties of Metals • An alloy is a solid or liquid mixture of two or more metals. • The properties of an alloy are different from the properties of the individual elements. • Often these properties eliminate some disadvantages of the pure metal. • A common alloy is brass, a mixture of copper and zinc. • Brass is harder than copper and more resistant to corrosion. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Comparing Metals, Metalloids, and Nonmetals Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. HOMEWORK SECTION REVIEW Pg. 131 Q(1-9) Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 1. Which group of elements is the most unreactive?Why? The noble gases ( group 18)because they have a full valence level of electrons 2. Why do groups among the main-group elements display similar chemical behavior? They have the same number of valence electrons 3. What properties do the halogens have in common? They have 7 valence electrons, highly reactive, react with many metals, particularly alkali metals to form salts 4. Why is hydrogen set apart by itself? It has one proton and one electron. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 5. How do the valence electron configurations of the alkali metals compare with each other? They have the same number of valence electrons in their outer s orbital. 6. Why are the alkaline-earth metals less reactive than the alkali metals? Alkaline earth metals must lose 2 electrons instead of one to become stable. 7. In which groups of the periodic table do the transition metals belong? Groups 3 through 12 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 8. Why are the nuclear structures of the actinides more important than the electron configurations of the actinides? Their nuclei are unstable and spontaneously break apart, making them radioactive 9. What is an alloy? A mixture of a metal and one or more other elements. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Bellringer • Draw atomic models of lithium, magnesium, and fluorine. • From the models, predict whether the ions of these elements will be larger or smaller than the atoms. Be sure to justify your predictions. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Objectives • Describe periodic trends in ionization energy, and relate them to the atomic structures of the elements. • Describe periodic trends in atomic radius, and relate them to the atomic structures of the elements. • Describe periodic trends in electronegativity, and relate them to the atomic structures of the elements. • Describe periodic trends in ionic size, electron affinity, and melting and boiling points, relate them to the atomic structures of the elements. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Periodic Trends • The arrangement of the periodic table reveals trends in the properties of the elements. • A trend is a predictable change in a particular direction. • Understanding a trend among the elements enables you to make predictions about the chemical behavior of the elements. • These trends in properties of the elements in a group or period can be explained in terms of electron configurations. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Ionization Energy • The ionization energy is the energy required to remove an electron from an atom or ion. A + ionization energy A + + e neutral atom ion electron Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Ionization Energy, continued Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. The elements of Group 1, the Alkali metals, are: symbol electron configuration lithium Li 1s22s1. sodium Na 1s2 2s22p6 3s1 potassium K 1s2 2s2 2p6 3s2 3p6 4s1 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Symbol of the electron configuration of the ion ion lithium ion Li + 1s22s1 1s2 Sodium ion Na + 1s2 2s22p6 3s1 1s2 2s22p6 potassiu m ion K+ 1s2 2s2 2p6 3s2 3p6 4s11s2 2s2 2p6 3s2 3p6 Least Energy most ionization energy Chapter menu less Energy Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Ionization Energy, continued Ionization Energy Decreases as You Move Down a Group • Each element has more occupied energy levels than the one above it has. • The outermost electrons are farthest from the nucleus in elements near the bottom of a group. • As you move down a group, each successive element contains more electrons in the energy levels between the nucleus and the outermost electrons. • Electron shielding is the reduction of the attractive force between a positively charged nucleus and its outermost electrons due to the cancellation of some of the positive charge by the negative charges of the inner electrons. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Ionization Energy, continued Ionization Energy Decreases as You Move Down a Group, continued Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Ionization Energy, continued Ionization Energy Increases as You Move Across a Period • Ionization energy tends to increase as you move from left to right across a period. • From one element to the next in a period, the number of protons and the number of electrons increase by one each. • The additional proton increases the nuclear charge. • A higher nuclear charge more strongly attracts the outer electrons in the same energy level, but the electron-shielding effect from inner-level electrons remains the same. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Write the electron configuration for each of the following elements. Sodium Na 1s2 2s22p6 3s1 Least ionization Energy Magnesium Mg 1s2 2s22p6 3s2 More ionization E Aluminum Al 1s2 2s22p6 3s2 3p1 More E Silicon Si 1s2 2s22p6 3s2 3p2 Most E Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Ionization Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Atomic Radius • The exact size of an atom is hard to determine. • The volume the electrons occupy is thought of as an electron cloud, with no clear-cut edge. • In addition, the physical and chemical state of an atom can change the size of an electron cloud. • One method for calculating the size of an atom involves calculating the bond radius, which is half the distance from center to center of two like atoms that are bonded together. • The bond radius can change slightly depending on what atoms are involved. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Atomic Radius, continued Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Bond Length Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Atomic Radius, continued Atomic Radius Increases as You Move Down a Group • As you proceed from one element down to the next in a group, another principal energy level is filled. • The addition of another level of electrons increases the size, or atomic radius, of an atom. • Because of electron shielding, the effective nuclear charge acting on the outer electrons is almost constant as you move down a group, regardless of the energy level in which the outer electrons are located. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Atomic Radius, continued Atomic Radius Decreases as You Move Across a Period • As you move from left to right across a period, each atom has one more proton and one more electron than the atom before it has. • All additional electrons go into the same principal energy level—no electrons are being added to the inner levels. • Electron shielding does not play a role as you move across a period. • As the nuclear charge increases across a period, the effective nuclear charge acting on the outer electrons also increases. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Periodic Trends of Radii Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Electronegativity • Not all atoms in a compound share electrons equally. • Knowing how strongly each atom attracts bonding electrons can help explain the physical and chemical properties of the compound. • Linus Pauling, an American chemists, made a scale of numerical values that reflect how much an atom in a molecule attracts electrons, called electronegativity values. • Electronegativity is a measure of the ability of an atom in a chemical compound to attract electrons. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Electronegativity, continued Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Electronegativity, continued • The atom with the higher electronegativity will pull on the electrons more strongly than the other atom will. • Fluorine is the element whose atoms most strongly attract shared electrons in a compound. Pauling arbitrarily gave fluorine an electronegativity value of 4.0. • Values for the other elements were calculated in relation to this value. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Electronegativity, continued Electronegativity Decreases as You Move Down a Group • Electronegativity values generally decrease as you move down a group. • The more protons an atom has, the more strongly it should attract an electron. • However, electron shielding plays a role again. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Electronegativity, continued Electronegativity Increases as You Move Across a Period • Electronegativity usually increases as you move left to right across a period. • As you proceed across a period, each atom has one more proton and one more electron—in the same principal energy level—than the atom before it has. • Electron shielding does not change as you move across a period because no electrons are being added to the inner levels. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Electronegativity, continued Electronegativity Increases as You Move Across a Period, continued • The effective nuclear charge increases across a period. • As this increases, electrons are attracted much more strongly, resulting in an increase in electronegativity. • The increase in electronegativity across a period is much more dramatic than the decrease in electronegativity down a group. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Electronegativity, continued Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Other Periodic Trends • The effective nuclear charge and electron shielding are often used in explaining the reasons for periodic trends. • Effective nuclear charge and electron shielding also account for two other periodic trends–ionic size and electron affinity. • The trends in melting and boiling points are determined by how electrons form pairs as d orbitals fill. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Other Periodic Trends, continued Periodic Trends in Ionic Size and Electron Affinity • Like atomic size, ionic size has periodic trends. • As you proceed down a group, the outermost electrons in ions are in higher energy levels. • The ionic radius usually increases as you move down a group. • This trends hold for both positive and negative ions. • Metals tend to lose one or more electrons and form a positive ion. • As you move across a period, the ionic radii of metal cations tend to decrease because of the increasing nuclear charge. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Other Periodic Trends, continued Periodic Trends in Ionic Size and Electron Affinity, continued • The atoms of nonmetal elements in a period tend to gain electrons and form negative ions. • As you proceed through the anions on the right of a period, ionic radii still tend to decrease because of the anions’ increasing nuclear charge. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Other Periodic Trends, continued Periodic Trends in Ionic Size and Electron Affinity Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Other Periodic Trends, continued Periodic Trends in Ionic Size and Electron Affinity, continued • The energy change that occurs when a neutral atom gains an electron is called the atom’s electron affinity. • This property of an atom is different from electronegativity. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. • The electron affinity tends to decrease as you move down a group because of the increasing effect of electron shielding. • Electron affinity tends to increase as you move across a period because of the increasing nuclear charge. Ionization energies are always concerned with the formation of positive ions. Electron affinities are the negative ion equivalent. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Other Periodic Trends, continued Periodic Trends in Ionic Size and Electron Affinity Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Electron Affinity Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Other Periodic Trends, continued Periodic Trends in Melting and Boiling Points Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Melting Point Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Boiling Point Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Additional Periodic Trends Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Additional Periodic Trends, continued Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 3 Trends in the Periodic Table Additional Periodic Trends, continued Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. HOMEWORK SECTION REVIEW PG. 141 Q 111 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 1. What is ionization energy? Is the amount of energy needed to remove an electron from an atom. 2. Why is measuring the size of an atom difficult? An atom has an electron cloud that has no definite boundary. 3. What can you tell about an atom that has high electronegativity? It will strongly attract other electrons in a compound. 4. How does electron shielding affect atomic size as you move down a group? Electron shielding contributes to the increase in atomic size as you move down a group. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 5. What periodic trends exist for ionization energy? Ionization energy decreases down a group and increases from left to right across a period. 6. Describe one way in which atomic radius is defined. Half the distance between the nuclei of two bonded atoms. 7. Explain how the trends in melting and boiling points differ from the other periodic trends. They are not consistent. Across a period they may increase, then decrease, then repeat the pattern. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. 8. Why do both atomic size and ionic size increase as you move down a group? Another principal energy level is added as you move from one element to the next, resulting in an increase in size. 9. How is electron affinity different from electronegativity? Electron affinity is the attraction an atom has when it is not bonded. Electronegativity is the attraction an atom has when it is bonded to another atom. 10. What periodic trends exist for electronegativity? Decreases down a group and increases from left to right 11. Why is electron shielding not a factor when you examine a trend across a period? Each atom across a period has the same number of energy levels and therefore the same amount of shielding. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Bellringer • Find technetium, promethium, and neptunium on a blank periodic table. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Objectives • Describe how the naturally occurring elements form. • Explain how a transmutation changes one element into another. • Describe how particle accelerators are used to create synthetic elements. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Natural Elements • Of all the elements listed in the periodic table, 93 are found in nature. • Three of these elements, technetium, Tc, promethium, Pm, and neptunium, Np, are not found on Earth but have been detected in the spectra of stars. • Most of the atoms in living things come from just six elements. • carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Natural Elements, continued Hydrogen and Helium Formed After the Big Bang • Much of the evidence about the universe’s origin points toward a single event: an explosion of unbelievable violence, before which all matter in the universe could fit on a pinhead. This event is known as the big bang. • As the universe expanded, it cooled and some of the energy was converted into matter in the form of electrons, protons, and neutrons. • As the universe continued to cool, these particles started to join and formed hydrogen and helium atoms. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Natural Elements, continued Hydrogen and Helium Formed After the Big Bang, continued • Gravity pulled these clouds of hydrogen closer and closer. • As the clouds grew more dense, pressures and temperatures at the centers of the hydrogen clouds increased, and stars were born. • In the centers of stars, nuclear reactions took place. • A nuclear reaction is a reaction that affects the nucleus of an atom. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Nuclear Reaction Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Nuclear Fusion Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Natural Elements, continued Other Elements Form by Nuclear Reactions in Stars • Einstein’s equation E = mc2 describes the mass-energy relationship quantitatively. • Einstein’s equation shows that fusion reactions release very large amounts of energy. • The energy released by a fusion reaction is so great it keeps the centers of the stars at very high temperatures. • During fusion in stars, two helium nuclei fuse to form a beryllium nucleus, and gamma radiation is released. • Repeated fusion reactions can form atoms as massive as iron and nickel. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Natural Elements, continued Other Elements Form by Nuclear Reactions in Stars, continued • Very massive stars (stars whose masses are more than 100 times the mass of our sun) are the source of heavier elements. • When such a star has converted almost all of its core hydrogen and helium into the heavier elements up to iron, the star collapses and then blows apart in an explosion called a supernova. • All of the elements heavier than iron on the periodic table are formed in this explosion. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Natural Elements, continued Other Elements Form by Nuclear Reactions in Stars Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Nuclear Fusion: Stellar Formation of Carbon-12 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Transmutations • In the Middle Ages, many early chemists tried to change, or transmute, ordinary metals into gold. • These early chemists did not realize that a transmutation, whereby one element changes into another, is a nuclear reaction. It changes the nucleus of an atom and therefore cannot be achieved by ordinary chemical means. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Visual Concepts Transmutation Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Transmutations, continued Transmutations Are a Type of Nuclear Reaction • Because of the results of his experiments, Ernest Rutherford believed that the nuclei in air had disintegrated into the nuclei of hydrogen (protons) plus the nuclei of some other atom. • W. D. Harkins and P.M.S. Blackett studied this strange phenomenon further. • They concluded that the Y formed when an alpha particle collided with a nitrogen atom in air to produce an oxygen atom and a proton, and that a transmutation had thereby occurred. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Synthetic Elements • Chemists have synthesized, or created, more elements than the 93 that occur naturally. • These are synthetic elements. • All of the transuranium elements, or those with more than 92 protons in their nuclei, are synthetic elements. To make them, one must use special equipment, called particle accelerators. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Synthetic Elements, continued The Cyclotron Accelerates Charged Particles • Many of the first synthetic elements were made with the help of a cyclotron, a particle accelerator, in which charged particles are given one pulse of energy after another, speeding them to very high energies. • The particles then collide and fuse with atomic nuclei to produce synthetic elements. • There is a limit to the energies that can be reached with a cyclotron and therefore a limit to the synthetic elements that it can make. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Synthetic Elements, continued The Cyclotron Accelerates Charged Particles Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Section 4 Where Did the Elements Come From? Synthetic Elements, continued The Synchrotron Is Used to Create Superheavy Elements • Once the particles have been accelerated, they are made to collide with one another to make superheavy elements, which have atomic numbers greater than 106. • Most superheavy elements exist for only a tiny fraction of a second. • Thirty seconds is a very long life span for a superheavy element. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 1. Which of the following elements is formed in stars? A. curium B. einsteinium C. gold D. mendelevium Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 1. Which of the following elements is formed in stars? A. curium B. einsteinium C. gold D. mendelevium Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 2. Why are the Group 17 elements, the halogens, the most reactive of the nonmetal elements? F. They have the largest atomic radii. G. They have the highest ionization energies. H. They are the farthest right on the periodic table. I. They require only one electron to fill their outer energy level. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 2. Why are the Group 17 elements, the halogens, the most reactive of the nonmetal elements? F. They have the largest atomic radii. G. They have the highest ionization energies. H. They are the farthest right on the periodic table. I. They require only one electron to fill their outer energy level. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 3. Which of the following is a property of noble gases as a result of their stable electron configuration? A. large atomic radii B. high electron affinities C. high ionization energies D. a tendency to form both cations and anions Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 3. Which of the following is a property of noble gases as a result of their stable electron configuration? A. large atomic radii B. high electron affinities C. high ionization energies D. a tendency to form both cations and anions Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 4. Which of these is a transition element? F. Ba G. C H. Fe I. Xe Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 4. Which of these is a transition element? F. Ba G. C H. Fe I. Xe Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 5. How did the discovery of the elements that filled the gaps in Mendeleev's periodic table increase confidence in the periodic table? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 5. How did the discovery of the elements that filled the gaps in Mendeleev's periodic table increase confidence in the periodic table? Answer: The gaps were significant because they predicted the properties of new elements that would be discovered. Their discovery demonstrated that the table was a useful tool for organizing information about atoms. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 6. Why is iodine placed after tellurium on the periodic table if the atomic mass of tellurium is less than that of iodine? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 6. Why is iodine placed after tellurium on the periodic table if the atomic mass of tellurium is less than that of iodine? Answer: Because the periodic table is based on atomic number, not atomic mass. The atomic number of iodine is one higher than the atomic number of tellurium. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 7. What is the outermost occupied energy level in atoms of the elements in Period 4? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Understanding Concepts 7. What is the outermost occupied energy level in atoms of the elements in Period 4? Answer: Level 4 Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills Read the passage below. Then answer the questions. The atomic number of beryllium is one less than that of boron, which follows it on the periodic table. Strontium, which is directly below beryllium in period 5 of the periodic table has 34 more protons and 34 more electrons than beryllium. However, the properties of beryllium resemble the much larger strontium more than those of similar-sized boron. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills 8. The properties of beryllium are more similar to those of strontium than those of boron because A. B. C. D. strontium is larger than boron. strontium and beryllium are both metals. strontium has more electrons than boron. strontium has the same number of valence electrons as beryllium. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills 8. The properties of beryllium are more similar to those of strontium than those of boron because A. B. C. D. strontium is larger than boron. strontium and beryllium are both metals. strontium has more electrons than boron. strontium has the same number of valence electrons as beryllium. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills 9. Beryllium and strontium are both located in the second column of the periodic table. To which of these classifications do they belong? F. G. H. I. alkali metals alkaline earth metals rare earth metals transition metals Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills 9. Beryllium and strontium are both located in the second column of the periodic table. To which of these classifications do they belong? F. G. H. I. alkali metals alkaline earth metals rare earth metals transition metals Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills 10. Why is it easier to determine to which column of the periodic table an element belongs than to determine to which row it belongs, based on observations of its properties? Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills 10. Why is it easier to determine to which column of the periodic table an element belongs than to determine to which row it belongs, based on observations of its properties? Answer: It is easier to determine the column because all the elements in a column have the same outer electron structure and, therefore, similar properties. Properties of elements across a row of the table vary widely. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Interpreting Graphics Use the diagram below to answer question 11. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Interpreting Graphics 11. What process is represented by this illustration? A. chemical reaction B. ionization C. nuclear fission D. nuclear fusion Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Interpreting Graphics 11. What process is represented by this illustration? A. chemical reaction B. ionization C. nuclear fission D. nuclear fusion Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Interpreting Graphics The graph below shows the ionization energies (kilojoules per mole) of main-block elements. Use it to answer questions 12 and 13. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Interpreting Graphics 12. Which of these elements requires the most energy to remove an electron? F. argon G. fluorine H. nitrogen I. oxygen Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Interpreting Graphics 12. Which of these elements requires the most energy to remove an electron? F. argon G. fluorine H. nitrogen I. oxygen Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills 13. Explain the trend in ionization energy within a group on the periodic table. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved. Chapter 4 Standardized Test Preparation Reading Skills 13. Explain the trend in ionization energy within a group on the periodic table. Answer: Ionization energy tends to increase from left to right across the table because elements have increasingly more protons so the attraction on the outer electrons is stronger. Chapter menu Resources Copyright © by Holt, Rinehart and Winston. All rights reserved.