* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download IASC Geneva Dec 2005 - Inter
Survey
Document related concepts
Transcript
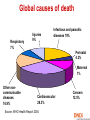
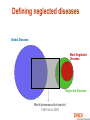
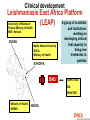
They need new drugs, vaccines and diagnosis now: reality of neglected diseases Bernard Pecoul Executive Director, DNDi Geneva 7 December 2005 Global causes of death Respiratory 7% Injuries 9% Infectious and parasitic diseases 19% Infectious and Perinatal 4.3% Parasitic diseases 33% Maternal 1% Other noncommunicable diseases 16.9% Cardiovascular 29.3% Source: WHO Health Report 2004 Cancers 12.5% Defining neglected diseases Global Diseases Most Neglected Diseases Neglected Diseases World pharmaceutical market > $518 bn in 2004 Sleeping sickness is a most neglected disease An estimated 300,000 infected 55 million at risk in sub-Saharan Africa Difficult to diagnose Fatal if untreated Existing drugs: old - toxic - resistance - difficult to use - expensive Source: WHO 2001 • • • • • The needs remain huge Arsenical Anti-cancer drug Leishmaniasis • • • • • An estimated 12 million people affected Different forms: visceral, (muco)cutaeous, PKDL 350 million people at risk in 88 countries Per year: 1-1.5 million new cases of CL/MCL 500,000 cases of VL VL is fatal if left untreated Existing drugs: old - toxic - resistance - difficult to use - expensive Buruli ulcer Source: WHO, WHO/CDS/CPE/GBUI/2001.1 AIDS is a neglected disease for adults and children living in developing countries • Drugs not adapted to health systems of endemic countries • No treatment adapted to children • Limited tools for diagnosis and follow up • No field-adapted preventive tools Treatments do not exist or are inadequate and inaccessible • • • • • Toxic Expensive Painful to deliver Difficult to follow up Not adapted to patient’s needs • Not registered in endemic regions • Restricted by patents Analysing the problems: Fatal imbalance Developing countries have a tiny share of the pharma market World Pharmaceutical Market, 2004: Total $518 billion Japan $58bn (11.1%) Asia, Africa and Australia $40bn (7.7%) Latin America $19bn (3.8%) Rest of Europe $9bn (1.8%) EU $144bn (27.8%) Source: IMS Health North America $248bn (47.8%) Only 1% of new drugs developed are for neglected diseases • Approx. 1-2% is spent on R&D for neglected diseases • 10/90 gap in health research spending • 1975-1999: 1,393 new chemical entities marketed Tropical diseases: 13 Tuberculosis: 3 Spending on health R&D has increased • World-wide spending on health R&D was never so high – Estimated at US$106bn for 2004 (GFHR, 2004) • Since 90’s: private sector has become biggest investor Bn US$ US-spending on health R&D: (>2/3rd total) 35.0 Government 30.0 Industry 25.0 20.0 15.0 10.0 5.0 Sources: For government: National Science Foundation 2004, http://www.nsf.gov/sbe/srs/nsf04329/pdf/nsf04329.pdf For Industry: PhRMA 2004, http://www.nsf.gov/sbe/srs/nsf04329/pdf/nsf04329.pdf 0.0 1980 1985 1990 1995 2000 2005 Gaps exist in the R&D process for neglected diseases… New knowledge on drug targets and lead compounds is published but pre-clinical research does not begin mainly industry (in North) GAP1 Discovery mainly public sector Pre Clinical Development GAP2 Validated candidate drugs do not enter clinical development because of strategic company choices. Availability to patients GAP3 New or existing drugs do not reach patients: registration problems, lack of production, high prices, or not adapted to the local conditions of use …due to failure of the market and public policy Market failure Drug development largely confined to the R&D-based pharmaceutical industry operating for profit Poorer patients are thus neglected Public policy failure Public policy does not redress this imbalance DNDi’s created in 2003: vision • Use an alternative model to develop new drugs for neglected diseases - leishmaniasis, sleeping sickness and malaria • Ensure equitable access of needs-driven products • Strengthen existing capacity in disease-endemic countries • Build public responsibility and leadership in addressing needs of these patients • Bring together the international community, public sector and pharmaceutical industry DNDi’s Founding Partners Medecins Sans Frontieres (MSF) Institut Pasteur, France WHO/TDR (permanent observer) Malaysian Ministry of Health Oswaldo Cruz Foundation, Brazil Kenya Medical Research Institute (KEMRI) Indian Council for Medical Research (ICMR) 18 projects in DNDi’s portfolio 2005 Discovery Nitroimidazoles project for trypanosomiasis Pharma • sanofi-aventis, France-Germany • Roche, CH • Chiron, USA • Novartis (NITD), CH Singapore • Romark, USA • Alkem, India Academics OBJECTIVE: To identify new drug candidates amongst old and new nitroimidazoles for trypanosomiasis • Swiss Tropical Institute • Fiocruz, Brazil • U of Sao Paolo, Brazil • U of Tehran, Iran • U of Bern, CH • Silesian University, Poland • Roma University, Italy other + contacts Japan, USA • TB alliance • Dr Nagarajan , India DNDi Pre-clinical development Ravuconazole project for Chagas disease Pharma companies Academic groups •Federal Univ of Ouro Preto, Brazil •Instituto Venezolano de Investigaciones Científicas, Venezuela DNDi Eisai, JAPAN OBJECTIVE: To investigate the activity and toxicity of ravuconazole in preclinical disease models for acute and chronic Chagas disease Clinical development Leishmaniasis East Africa Platform A group of scientists •University of Khartoum (LEAP) •Federal Ministry of Health •MSF- Holland SUDAN •Addis Ababa University •DACA •Ministry of Health ETHIOPIA DNDi and institutions working on developing clinical trial capacity to bring new treatments to patients IOWH- India IDA WHO/TDR •Ministry of Health •KEMRI KENYA Agreement between DNDi and sanofi-aventis Governments should tackle this imbalance • The response should not be purely philanthropic • Governments should increase public responsibility towards R&D of drugs for neglected diseases – More political leadership – Sustained financial support – New rules to stimulate drug R&D Increased public responsibility: 1. More public leadership • Make global health and medicines a strategic priority • Set R&D agenda according to the needs of patients Increased public responsibility: 2. Sustained financial support Governments need to • Raise current levels of funding for neglected diseases by 3 billion euros per year to start to correct the 10/90 gap • Put in place new, sustainable funding mechanisms 2. Sustained financial support This funding should be focused on: A needs-driven R&D agenda for safe, effective, affordable and field-adapted treatments Encouraging scientific community to do basic research on neglected diseases Translation of basic research to new medical applications, e.g. by supporting PDPs Encouraging R&D capacity strengthening in disease-endemic countries Securing the market Increased public responsibility: 3. New rules to stimulate drug R&D • Regulatory standards • Streamline regulatory approval processes to • • rapidly deliver essential medicines to patients Analyse risks and benefits of each drug or vaccine in relation to the needs of patients, severity of the disease and lack of alternative solutions Regulatory authorities (FDA and EMEA) should provide support and transfer knowhow to authorities in developing countries 3: New rules to stimulate drug R&D Intellectual Property: develop drugs as public goods • Guarantee that the public sector develops open access to information (recent Wellcome Trust policy) => open source (Human Genome Project) => freedom to operate • Ensure that industry provides sustainable access to knowledge, chemical compounds and tools • Make technology transfer happen to diseaseendemic countries www.dndi.org