* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Soil-Forming Factors
Survey
Document related concepts
Transcript
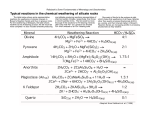
What should you know? Soil-Forming Factors ESS 210 Chapter 2 pages 31–74 • Weathering processes - physical and chemical • The five soil forming factors • Types of soil parent materials • Types of rocks and minerals • Impacts of parent material, climate, organisms, topography, and time on soil formation Primary Minerals Minerals • Light colored aluminosilicate minerals • Homogeneous, inorganic compounds, with definite chemical formula • Primary minerals – Quartz [SiO2]: most common, weather very slowly, sand size – Feldspars: sand size, weather to soil clays • K-feldspars – KAlSi3O8 • Plagioclase feldspars: – Formed as molten lava cools and solidifies – Not chemically altered by weathering processes • Secondary minerals – Albite – NaAlSi3O8 – Anorthite – CaAl2Si2O8 – Muscovite mica – KAl3Si3O10(OH)2 • A parent of soil clay minerals: weathers to soil clay minerals • Thin, translucent sheets (isinglass) – Recrystallization and/or alteration products of primary minerals Primary Minerals Secondary minerals • Dark colored, ferro-magnesium minerals – Biotite mica – KAl(Mg,Fe)3Si3O10(OH)2 • Thin dark sheets • Weathers to soil clay minerals – Hornblende – NaCa2Mg5Fe2AlSi7O22(OH) – Diopside – CaMgSi2O6 • Hornblende and diopside weather to soil clay minerals – Olivine – (Mg,Fe,Mn)2SiO4 • Ferro-magnesium minerals weather more rapidly than aluminosilicate minerals • Al and Fe (metal) oxides and hydroxides (sesquioxides) – – – – Goethite – FeOOH Hematite – Fe2O3 Gibbsite – Al(OH)3 Very stable soil minerals – dominate in OLD soils • Aluminosilicate clay minerals – several types, common, and chemically complex • Salts: calcite [CaCO3], gypsum [CaSO4•2H2O] 1 Rock Cycle Rocks Liquid Magma • Mixtures of minerals – Randomly dispersed, individual mineral crystals; heterogeneous solid • Texture refers to the size of mineral crystals in rock: fine, intermediate, coarse • Minerals present and rock texture determine weathering rate Igneous Rocks Heat & Pressure Cooling & Crystallization Heat & Pressure Igneous Weathering Metamorphic Weathering Heat & Pressure Sedimentary Igneous Rocks • Formed when molten lava cools • Primary minerals • Coarse textured: granite – Primarily quartz, feldspars, some dark minerals – very slow weathering • Fine to intermediate texture: basalt – hornblende, augite, biotite, and other dark minerals – relatively rapid weathering Sedimentary and Metamorphic Rocks Granite Basalt Sedimentary Rocks • Sedimentary: deposition and re-cementation of weathering products from other rocks – Sandstone, shale, limestone… • Metamorphic: igneous or sedimentary rocks transformed by high heat and/or pressure Granite Shale Gneiss, schist Slate Sandstone Quartzite Limestone Marble Sandstone Limestone 2 Metamorphic Rocks Weathering • The (1) physical disintegration of rock to form smaller rocks or individual mineral particles and the (2) chemical decomposition of minerals to form dissolved substances and new minerals • Weathering categories Gneiss Slate Physical Weathering A disintegration process that decreases particle size and increase particle surface area. Occurs through the affect of: • Temperature – Differential heating or cooling of rocks → exfoliation – Freeze-thaw: water expands upon freezing, exerting tremendous force • Abrasion by water and water-borne sediments, windblown particles, and ice in glaciers • Organisms – Plant roots – Soil animals – Humans Chemical Weathering Processes • Solutioning (dissolution): mineral dissolves in soil solution; common to soluble salts – CaSO4•2H2O (gypsum) → Ca2+ + SO42- + 2H2O – CaCO3 (calcite) → Ca2+ + CO32- • Hydrolysis: water acts upon a substance to create a new substance – Involves both H2O and H+ as reactants – Often results in release of nutrients from minerals and the formation of sesquioxides – KAlSi3O8 (K-feldspar) + 7 H2O + H+ → K+ + Al(OH)3 (gibbsite) + 3 H4SiO40 • Hydration: addition of water to a mineral structure – Physical – Chemical Chemical Weathering • Alters the composition of minerals • Conversion of primary minerals into secondary minerals, and secondary into other secondary minerals • Most rapid with warm temperatures, high precipitation, and small particle size • There are geochemical and biochemical agents of change • Water is required Chemical Weathering Processes Hydrolysis is an important weathering process • Presence of H+ (acidity) accelerates weathering • Sources of protons – CO2 in rainfall produces carbonic acid: CO2 + H2O → H2CO3 → H+ + HCO3– (rainfall is naturally acidic; pH ~ 5.6) – Plant roots and soil organisms respire and produce carbonic acid – Soil organic matter is a proton source – Other acidic substances in rainfall: SOx/NOx + H2O → H2SO4/HNO3 – Fertilizers (e.g., NH4+) – 5 Fe2O3 (hematite) + 9H2O → Fe10O15•9H2O (ferrihydrite) 3 Chemical Weathering Processes • Oxidation/reduction (redox) reactions (the second most important weathering process) (e–) – Addition or loss of electrons from atom in a mineral – Oxidation = loss of e–; reduction = gain of e– – Electron-rich elements are termed reduced (e.g., Fe2+); electron-poor elements are termed oxidized (e.g., Fe3+) – O2 is most common oxidizing agent – Elements in primary minerals commonly exist in a reduced state – Oxidation and reduction occur together; they are coupled Complexation Reactions • Microorganisms and plant roots exude organic acid anions, e.g., citrate, oxalate, and malate • These organic acids bond with (chelate) metals, e.g., Al3+ and Fe3+, to form soluble complexes • The metal-organic complex is stable and much more soluble than the metal ion alone Soil Formation Processes • Soil is an open system • Additions - movement into profile – – – – Organic matter Rainfall Sediments Chemicals: natural and anthropogenic • Losses - movement out of profile – – – – – Evapotranspiration Erosion Leaching of water and chemicals Gaseous losses of nutrients Removal by vegetation Redox Reactions • Oxidation of Fe2+ by O2 (O2 is the oxidant, it will be reduced during the redox process) • Oxidation half-reaction: Fe2+ → Fe3+ + e– • Reduction half-reaction: ¼O2 + e– + H+ → ½H2O • Complete redox reaction: Fe2+ + ¼O2 + H+ → Fe3+ + ½H2O Complexation Reactions Example: Al3+ complexation by ketogluconate Al(OH)3 (gibbsite) + 3H+ → Al3+ + 3H2O Al3+ + C5O5H9COO– → C5O5H8COOAl+ + H+ Soil Formation Processes • Translocations: movement within the soil profile – Eluvial processes – Illuvial processes • Transformations: a change in form – Physical weathering – Chemical weathering – Microbial degradation 4 Five Soil Forming Factors • Soil is a dynamic natural body formed by the combined effects of climate and biota, as moderated by topography, acting on parent materials over time. • Soil = ƒ(climate, biota, topography, parent materials, time) Residual Parent Materials • Soils develop from underlying bedrock – Igneous, sedimentary, metamorphic • Type of rock strongly influences type of soil – Limestone → clayey soils – Sandstone → coarse, acidic soils – Granite → coarse, acidic soils – Slate, shale → clayey soils Factor One: Parent Material • Parent material impacts – Soil textural class – Innate soil fertility – Types of clay minerals – Soil pH • Classes of parent materials based on placement – Residual – Transported (six types of transported materials) Transported Parent Materials • • • • • • Colluvial debris Alluvial deposits Marine sediments Lacustrine sediments Eolian deposits Glacial deposits Colluvial Debris • Poorly sorted fragments on steep slopes or at the foot of slopes, carried by gravity • Small geographical areas • Usually rocky and stony, no layering • Physical weathering processes dominate relative to chemical weathering processes • Well-drained but unstable 5 Alluvial Deposits • Floodplains – During flooding, water spreads and slows, and fine sediment is deposited. – Horizontal and vertical stratification – Terraces are old floodplains above the current floodplain – Usually very fertile soils and important for agriculture, forestry, wildlife – Poor choice for homes and other urban development Alluvial Deposits • Alluvial fans – Usually gravelly/stony in mountainous regions, can have finer material as well. – Stream leaves narrow upland channel, descends to broad valley below Alluvial Deposits • Delta deposits – The continuation/terminus of the floodplain – Rivers carry much clay/fine silt to lake or ocean – Very slow water = deposition of fine particles – Very clayey, swampy, poorly drained – Example: Mississippi River delta in Louisiana 6 Marine and Lacustrine Sediments • Marine - Coastal Plains – Ocean sediments build up over time – Exposed by changes in elevation of earth’s crust – Materials are gravely, sandy, clayey depending on area – Atlantic and Gulf Coastal areas, ~ 10% of US • Lacustrine – Lake sediments build up over time – Clayey soils formed as lakes dried – Major areas of lacustrine soils in glaciated areas Glacial Till • As glacier advances, grinds up rock and carries it • Till is unsorted, unconsolidated material • Deposited as glacier melts and recedes • Till deposits called moraines Eolian Deposits • Loess deposits – Common in central United States – Wind carried silts (coarse clays to fine sands) from glaciated areas – Cover other soils or parent materials – Western one-third of Tennessee is loessial – Very thick (8+ m) at Mississippi River to non-existent at Tennessee River – Blankets much of Iowa, thick at the Missouri River, thin on eastern side • Others - sand dunes (sand-size), aerosolic dust (clay-size), volcanic ash (allophanic soils) Ground moraines Terminal moraines – Ground moraine - material deposited in relatively uniform layer during retreat – Terminal or end moraine - material left pushed up in ridge at southern-most edge of advancing glacier – Recessional moraine – terminal moraines from more than one advance Glacial Outwash Factor Two: Climate • As glaciers melt, glacial rivers and streams form and carry sediments Influences soil formation three ways: 1. Precipitation 2. Temperature 3. Native Vegetation – Coarse materials drop first – Fine materials carried furthest • Deposits are sorted 7 Climate: Precipitation • As rainfall increases, chemical and physical weathering rates increase • Profile depth increases • Nutrient status changes – Loss of base cations Ca2+, Mg2+, K+, Na+ – Al3+, Fe3+, Mn2+, H+ increase • Soil acidity increases Soil Moisture Regimes • • • • • Aquic = wet = tile needed for row crops Udic = enough precipitation for “corn” Ustic = enough precipitation for “wheat” Aridic = cacti without irrigation Xeric = precipitation when not needed for production of most crops → winter Soil Temperature Regimes • Cryic – mean annual T < 8 ºC • Frigid – mean annual T < 8 ºC; difference between mean summer and mean winter T is > 6 ºC • Mesic – mean annual T > 8 ºC and < 15 ºC; difference between mean summer and mean winter T is > 6 ºC • Thermic – mean annual T > 15 ºC and < 22 ºC; difference between mean summer and mean winter T is > 6 ºC • Hyperthermic – mean annual T > 22 ºC; difference between mean summer and mean winter T is > 6 ºC Soil Moisture Regimes • Aquic: saturated with reducing conditions most of the year • Udic: soil moisture control section is dry for < 90 cumulative days per year • Ustic: is dry for > 90 cumulative days per year • Aridic: dry in all parts for > half the year • Xeric: moist winters, dry summers (Mediterranean, California) Climate: Temperature • Chemical and biological reaction rates double for every 10 ºC increase • Climates with extreme T, physical weathering (e.g., freeze-thaw) more significant than chemical weathering • Evapotranspiration increases with increasing T Climate: Type of vegetation • Humid = forest • Sub-humid, semi-arid = grasslands • Arid = shrubs, brush, succulents 8 Factor Three: Biota • Plants, animals, microorganisms • Important for MANY processes in soil formation • Chemical weathering – Organic acid anions, carbonic acid, oxidationreduction • Organic matter accumulation (humification) – Water holding, nutrient holding • Nutrient cycling – Base recycling – Ca, Mg, K • Nitrogen addition – Microbial N-fixation – N2 → NH4+ • Profile mixing • Aggregation – bioturbation – earthworms, insects, etc. – Polysaccharides, gelatinous materials Impact of Native Vegetation • Grasslands Impact of Native Vegetation • Deciduous forests – High OM below surface – Continuous root production, high interception of rain • Coniferous Forests – Vegetation low base cations (Ca, Mg, K) – Low recycling – Highly leached, acidic soils Grassland vs. Forest Soils Grassland Biota Deciduous Coniferous – High in basic cations – High base cycling – Slightly to moderately acid • Forest soils are usually more “developed” with more horizons, etc... Factor Four: Topography • Affects amount of water soil “sees” (yellow arrows): concept of “effective precipitation” • Slope aspect affects soil temperature Footslope Upland stable Sideslope active deposition active erosion Mollisol Alfisol Spodosol Floodplain active deposition Terrace/Fan stable 9 Landscape Positions Landscape Positions • Upland • Terrace (second bottom, bench land) – Soil developed in residuum or in stable, unconsolidated materials (loess, glacial till) – Rocks angular (except in till) – Well-developed soils – Highly-dissected • Footslope – Bottom of slope, colluvial and alluvial deposits – Partly rounded rock, immature/younger soils Topography: Catena or Toposequence Soils with same parent material, differ primarily in topographic location – Old alluvium, higher elevation than current Floodplain – Round stones, rocks - indicates water worked – Mature soils, some dissection • Bottomland (floodplain) – Deposited by present stream action – Rounded stones – Immature soils, little dissection Hawthorne-Dellrose-Mimosa Inceptisol A AE Bw C Ultisol Alfisol Typical pattern of soils and underlying material in the HawthorneDellrose-Mimosa general soil map unit (Marshall Co., TN) A BA A Bt1 Bt2 Bt3 Bt4 BC C Bt1 Cr Bt2 2Bt3 R Hawthorne Dellrose Mimosa Factor Five: Time • Pretty obvious! • Works in concert with other factors • Chronologically old soil may be developmentally young, e.g., arid region soils which have very little development • Soil “age” is a relative thing! • “Old” soils = high water throughput (Ultisols & Oxisols) • “Young” soils = low water throughput (Aridisols) Physiography of Tennessee Central Basin Mississippi River Great Valley Plateau Slope Highland Rim Unaka Range Cumberland Plateau Modified from "Geography of Tennessee", published by Ginn and Co. 10 Physiographic Regions Mississippi River floodplain Loess Coastal Plain Highland Rim Central Basin Cumberland Plateau Valley and Ridge Smoky Mountains Regions and their soils • Cumberland Plateau – Generally loamy soils – Sandstone is dominant parent material – Ultisols dominant • Highland Rim – Generally clayey soils, many cherty – Limestone is dominant parent material – Ultisols and Alfisols • Central Basin – Clayey, often shallow soils – Alfisols, Ultisols, Mollisols, Inceptisols Regions and their soils • Unaka Range – Generally young (developmentally), shallow soils. – Parent materials are metamorphic and igneous rock – Inceptisols very common - weak horizonation – Ultisols in valleys, low elevations • Valley and Ridge region (Knoxville) – Well-developed soils – Ultisols and Alfisols in limestone, sandstone, shale Regions and their soils • Coastal Plain – Ultisols & Alfisols – Clayey soils from fine sediments – Loamy soils in coarse sediments – Fine-loamy soils in loess over sediments • West Tennessee Loess Region - Alfisols – Fine-loamy soils in loess deposits, many fragipans – Erosion is major risk • Mississippi River Floodplain – Entisols, Inceptisols, Alfisols, Mollisols – Young, productive soils 11