* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download SLCO1B1 polymorphism markedly affects the pharmacokinetics of
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Prescription costs wikipedia , lookup
Hyaluronic acid wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
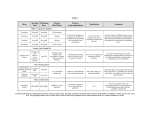
Original article 873 SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid Marja K. Pasanen, Mikko Neuvonen, Pertti J. Neuvonen and Mikko Niemi Background and objective Organic anion transporting polypeptide 1B1 (OATP1B1) is an uptake transporter located at the sinusoidal membrane of human hepatocytes. This study aimed to investigate the effects of genetic polymorphism in the SLCO1B1 gene encoding OATP1B1 on the pharmacokinetics of simvastatin. Methods Four healthy volunteers with the homozygous SLCO1B1 c.521CC genotype, 12 with the heterozygous c.521TC genotype and 16 with the homozygous c.521TT genotype (controls) were recruited. Each study participant ingested a single 40-mg dose of simvastatin. Plasma concentrations of simvastatin (inactive lactone) and its active metabolite simvastatin acid were measured for 12 h. Results The AUC0–N of simvastatin acid was 120 and 221% higher in participants with the SLCO1B1 c.521CC genotype than in those with the c.521TC and c.521TT (reference) genotypes, respectively (P < 0.001). The Cmax of simvastatin acid was 162 and 200% higher in participants with the c.521CC genotype than in those with the c.521TC and c.521TT genotypes (P < 0.001). The Cmax of simvastatin acid occurred earlier in participants with the c.521CC and c.521TC genotypes than in those with the c.521TT genotype (P < 0.05). No association existed between the SLCO1B1 genotype and the elimination half-life of simvastatin acid. Moreover, no statistically significant association was seen between the SLCO1B1 genotype and the pharmacokinetics of simvastatin lactone. Introduction Simvastatin is a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor used in the treatment of hypercholesterolemia [1]. It reduces cardiovascular morbidity and mortality in high-risk patients [2]. Simvastatin is administered as an inactive lactone prodrug, which undergoes reversible non-enzymatic and carboxylesterase-mediated conversion in plasma, liver and intestinal mucosa to simvastatin acid, the active metabolite of simvastatin [1,3]. Simvastatin acid and other simvastatin-derived active metabolites inhibit the conversion of HMG-CoA to mevalonate, which is the rate limiting step in cholesterol biosynthesis [4]. Inhibition of the HMG-CoA reductase in the liver increases the expression of low-density lipoprotein (LDL) receptors in the hepatocyte plasma membrane, enhancing the removal of LDL particles from blood and reducing plasma total and LDL cholesterol concentrations. Conclusions SLCO1B1 polymorphism markedly affects the pharmacokinetics of active simvastatin acid, but has no significant effect on parent simvastatin. Raised plasma concentrations of simvastatin acid in patients carrying the SLCO1B1 c.521C variant allele may enhance the risk of systemic adverse effects during simvastatin treatment. In addition, reduced uptake of simvastatin acid by OATP1B1 into the liver in patients with the c.521C allele could reduce its cholesterol-lowering efficacy. Pharmacogenetics and c 2006 Lippincott Williams & Genomics 16:873–879 Wilkins. Pharmacogenetics and Genomics 2006, 16:873–879 Keywords: OATP1B1, organic anion transporting polypeptide, pharmacogenetics, pharmacokinetics, simvastatin, simvastatin acid, SLCO1B1 Department of Clinical Pharmacology, University of Helsinki and Helsinki University Central Hospital, Helsinki, Finland. Correspondence and requests for reprints to Mikko Niemi, MD, Department of Clinical Pharmacology, Helsinki University Central Hospital, P.O. Box 340, FIN-00029 HUS, Finland. Fax: + 358 9 471 74039; e-mail: [email protected] Sponsorship: This study was supported by grants from the Helsinki University Central Hospital Research Fund (Helsinki, Finland) and the Sigrid Juselius Foundation (Helsinki). Received 2 May 2006 Accepted 20 June 2006 Large interindividual variability exists in the plasma concentrations of simvastatin and in its efficacy and risk of adverse effects. Myopathy is a relatively common adverse effect of statins, whereas the serious rhabdomyolysis is rare [5,6]. The incidence of myopathy and rhabdomyolysis increases along with increased plasma concentrations of statins, and particularly when statins are used concomitantly with compounds that inhibit their metabolism or plasma membrane transport [7,8]. Simvastatin undergoes extensive first-pass metabolism in the intestinal wall and the liver, and its systemic bioavailability is less than 5% [3]. Oxidative metabolism of simvastatin lactone in the liver is catalyzed mainly by cytochrome P450 (CYP) 3A4, with CYP3A5 contributing to a lesser extent [9]. Active simvastatin acid is also metabolized mainly by CYP3A4, with contribution from CYP3A5, CYP2C8 and UDP-glucuronosyltransferase c 2006 Lippincott Williams & Wilkins 1744-6872 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 874 Pharmacogenetics and Genomics 2006, Vol 16 No 12 [10,11]. CYP3A4 inhibitors, such as itraconazole, verapamil, erythromycin and grapefruit juice, markedly elevate the plasma concentrations of both simvastatin and simvastatin acid [12–14]. In addition, genetic polymorphism in CYP3A5 was associated with simvastatin efficacy in one study [15]. Organic anion transporting polypeptide 1B1 (OATP1B1) is a solute carrier expressed on the sinusoidal membrane of human hepatocytes [16–18]. It facilitates the hepatic uptake of many endogenous and foreign compounds, such as estrogen conjugates, bile acids and statins [16–24]. A common single nucleotide polymorphism (SNP) in SLCO1B1, c.521T > C (p.Val174Ala), has been associated with reduced activity of OATP1B1 in vitro [23,25] and markedly increased plasma concentrations of pravastatin, rosuvastatin and pitavastatin in vivo in humans [26–30]. In one study performed in vitro, simvastatin lactone was not transported by OATP1B1 [23], but there seem to be no studies directly investigating whether simvastatin acid is a substrate of OATP1B1. Simvastatin acid, however, strongly inhibits OATP1B1-mediated uptake of pravastatin in vitro, suggesting that it may also be a substrate for OATP1B1 [17,31]. As SLCO1B1 polymorphism markedly affects the pharmacokinetics of several statins, we wanted to investigate the effects of variant SLCO1B1 Table 1 genotypes on the pharmacokinetics of simvastatin and simvastatin acid in a prospective genotype panel study controlling for CYP3A5 polymorphism. Methods Study participants A total of 32 young healthy Caucasian volunteers participated in the study after giving written informed consent. The study participants had been genotyped for SLCO1B1 SNPs by TaqMan allelic discrimination with an Applied Biosystems 7300 Real-Time PCR system (Applied Biosystems, Foster City, California, USA) as described previously [32]. In addition, they were genotyped for the CYP3A5*3 (g.6986A > G) allele as described previously [15]. As the CYP3A5 genotype has been associated with the efficacy of simvastatin [15], only participants with the CYP3A5 non-expressor genotype (CYP3A5*3/*3) were recruited. The participants were selected on the basis of the SLCO1B1 c.521T > C SNP as well as the g. – 11187G > A, g. – 10499A > C and c.388A > G SNPs, by which the four major Caucasian haplotypes containing the c.521C allele (*5, *15, *16 and *17) can be distinguished [28,32]. The participants were allocated into one of three groups according to the genotype. Haplotypes were assigned as described previously (Table 1) [32]. The control group included Genetic background of the study participants SLCO1B1 Participant no. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28b 29 30 31 32 g. – 11187G > A g. – 10499A > C c.388A > G c.521T > C Diplotypea GA GA GG GG GA GA GA GA GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG GG AC AC CC AA AA AA AA AA AC AC AC AC AA AA AA AA AA AA AA AA AA AA AA AA AA AA AA AA AA AA AA AA GG GG GG AG AG AG GG AG GG AG GG AG GG AG GG AG AA AA AA AA AA AA AA AA AA AA AA AA AA AA AA AA CC CC CC CC TC TC TC TC TC TC TC TC TC TC TC TC TT TT TT TT TT TT TT TT TT TT TT TT TT TT TT TT SLCO1B1*16/*17 SLCO1B1*16/*17 SLCO1B1*16/*16 SLCO1B1*5/*15 SLCO1B1*1A/*17 SLCO1B1*1A/*17 SLCO1B1*1B/*17 SLCO1B1*1A/*17 SLCO1B1*1B/*16 SLCO1B1*1A/*16 SLCO1B1*1B/*16 SLCO1B1*1A/*16 SLCO1B1*1B/*15 SLCO1B1*1A/*15 SLCO1B1*1B/*15 SLCO1B1*1A/*15 SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A SLCO1B1*1A/*1A a Diplotype prediction is based on the g. – 11187G > A, g. – 10499A > C, c.388A > G and c.521T > C single nucleotide polymorphisms: *1A haplotype is GAAT, *1B is GAGT, *5 is GAAC, *15 is GAGC, *16 is GCGC, *17 is AAGC. b This participant was a tobacco smoker. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. SLCO1B1 polymorphism and simvastatin Pasanen et al. 875 16 participants (eight women, eight men) with the homozygous reference genotype at each position (c.521TT group). Their mean ± SD age was 23 ± 2 years, height 174 ± 9 cm and weight 68 ± 10 kg. The second group consisted of 12 participants (five women, seven men) heterozygous for the c.521T > C SNP (c.521TC group). Their mean ± SD age was 24 ± 4 years, height 174 ± 9 cm and weight 69 ± 8 kg. The third group comprised four participants (one woman, three men) with the homozygous c.521CC genotype (c.521CC group). Their mean ± SD age was 23 ± 2 years, height 180 ± 8 cm and weight 84 ± 8 kg. The participants were ascertained to be healthy by medical history, physical examination and routine laboratory tests before they were entered in the study. One participant with the c.521TT genotype was a tobacco smoker and none used any continuous medication. Study design The study protocol was approved by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District and by the National Agency for Medicines. Following an overnight fast, the participants ingested a single 40-mg dose of simvastatin (Zocor, Merck Sharp & Dohme, Haarlem, The Netherlands) with 150 ml water at 08:00 h. A standard warm meal was served 4 h after simvastatin ingestion and a standard light meal after 7 and 10 h. Timed blood samples (5–10 ml each) were drawn before and 0.5, 1, 1.5, 2, 3, 4, 5, 7, 9 and 12 h after simvastatin ingestion into tubes that contained ethylenediaminetetraacetic acid and were placed on ice immediately after sampling. Plasma was separated within 30 min after blood sampling and stored at – 701C until analysis. Use of other drugs was prohibited for 1 week and use of grapefruit products was prohibited for 3 days before simvastatin administration. Determination of plasma simvastatin and simvastatin acid concentrations Plasma simvastatin and simvastatin acid concentrations were quantified by liquid chromatography-ion spray tandem mass spectrometry using the PE Sciex API 3000 LC/MS/MS system (Sciex Division of MDS Inc., Toronto, Ontario, Canada) as described previously [33,34]. The limit of quantification was 0.05 ng/ml for simvastatin and 0.1 ng/ml for simvastatin acid. The between-day coefficients of variation were 4.1% at 0.1 ng/ml, 5.2% at 1.0 ng/ml and 3.2% at 50 ng/ml of simvastatin (n = 4), and 9.9% at 0.1 ng/ml, 7.3% at 1.0 ng/ ml and 7.0% at 50 ng/ml of simvastatin acid (n = 4). Pharmacokinetics The pharmacokinetics of simvastatin and simvastatin acid were characterized by the peak concentration in plasma (Cmax), time to Cmax (tmax), elimination half-life (t1/2) and area under the plasma concentration–time curve from 0 to 12 h (AUC0–12) and from 0 h to infinity (AUC0–N). The terminal log-linear part of each concentration–time curve was identified visually, and the elimination rate constant (ke) was determined from log-transformed data with linear regression analysis. The t1/2 was calculated by the equation t1/2 = ln 2/ke. AUC was calculated by a combination of the linear and log-linear trapezoidal rules, with extrapolation to infinity, when appropriate, by division of the last measured concentration by ke. Statistical analysis Results are expressed as mean ± SD in the text and tables and, for clarity, as mean ± SEM in Fig. 1. The pharmacokinetic variables of simvastatin and simvastatin acid between the SLCO1B1 c.521TT, c.521TC and c.521CC genotype groups were compared using analysis of variance and pairwise testing with the Tukey test. The tmax data were analyzed by the Kruskal–Wallis test and pairwise testing with the Mann–Whitney U-test using the Bonferroni correction. The data were analyzed with the statistical program SPSS 11.0 for Windows (SPSS Inc., Chicago, Illinois, USA). On the basis of previous data on the pharmacokinetics of simvastatin [12], the number of participants in each genotype group was estimated to be sufficient to detect a 50% larger AUC0–N of simvastatin or simvastatin acid in participants with the c.521TC genotype than in those with the c.521TT genotype, and a 100% larger AUC0–N in participants with the c.521CC genotype than in those with the c.521TT genotype with a power of at least 80% (a-level 5%). Differences were considered statistically significant at a P value less than 0.05. Results The SLCO1B1 genotype was significantly associated with the pharmacokinetics of simvastatin acid, but not with those of the parent simvastatin lactone (Fig. 1a–c, Table 2). The simvastatin acid Cmax and AUC0–N values of one participant (no. 6) with the c.521TC genotype were more than 3 SDs above the respective mean values (Fig. 2a and c), and the participant was excluded from statistical analysis as an outlier. In the participants with the SLCO1B1 c.521CC genotype, the mean AUC0–N of simvastatin acid was 120 and 221% higher, respectively, than in the participants with the c.521TC and c.521TT (reference) genotypes (P < 0.001). The mean Cmax of simvastatin acid was 162 and 200% higher in the participants with the c.521CC genotype than in those with the c.521TC and c.521TT genotypes (P < 0.001). In addition, the tmax of simvastatin acid was significantly shorter in the participants with the c.521TC and c.521CC genotypes than in those with the c.521TT genotype (P < 0.05). The SLCO1B1 genotype had no statistically significant effect on the elimination t1/2 of simvastatin acid (Fig. 2b, Table 2). The mean AUC0–N ratio (simvastatin acid/simvastatin) was 1.5 ± 0.4 in the participants with the c.521CC Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 876 Pharmacogenetics and Genomics 2006, Vol 16 No 12 genotype, 0.7 ± 0.4 in those with the c.521TC genotype and 0.7 ± 0.2 in those with the c.521TT genotype (c.521CC vs. c.521TC, P = 0.001; c.521CC vs. c.521TT, P < 0.001) (Fig. 1c). A tendency towards higher Cmax and AUC0–N of simvastatin lactone was observed in the participants with the homozygous c.521CC genotype than in the reference group, but the differences were not statistically significant. When the outlier participant was included in the analysis, other differences remained statistically significant except for those in the AUC0–N ratio and the simvastatin acid Cmax between the c.521CC and c.521TC groups (data not shown). Fig. 1 (a) Simvastatin (ng/ml) 12 10 8 6 4 2 0 0 1 2 3 4 5 7 Time (h) 9 12 (b) 7 Simvastatin acid (ng/ml) 6 5 4 3 2 1 0 0 1 2 3 4 5 7 Time (h) 9 12 (c) 7 The Cmax and AUC0–N of simvastatin lactone varied eight-fold and 12-fold between individual participants and those of simvastatin acid varied 25-fold and 16-fold. The variability in the Cmax and AUC0–N of simvastatin acid, however, was considerably smaller within the SLCO1B1 c.521TT (six-fold and four-fold) or c.521CC (1.5-fold and 1.7-fold) genotype groups (Fig. 2a–c). The variability was large within the c.521TC group (17-fold and 14-fold), but this was because the outlier participant had exceptionally high values. Discussion 6 Simvastatin acid/simvastatin No statistically significant differences were seen in the pharmacokinetic variables of simvastatin acid between c.521TC heterozygous participants with different SLCO1B1 haplotypes. The mean ± SD AUC0–N of simvastatin acid was 25.0 ± 15.3 ng h/ml in c.521TC heterozygotes with the *15 haplotype, 19.0 ± 4.0 ng h/ml in those with the *16 haplotype and 36.5 ± 43.1 ng h/ml in those with the *17 haplotype (including the outlier) (P = 0.650). 5 4 3 2 1 0 0 1 2 3 4 5 7 9 12 Time (h) Mean ± SEM plasma concentrations of simvastatin (a) and simvastatin acid (b), and the plasma simvastatin acid/simvastatin ratio (c) after a single 40-mg oral dose of simvastatin in 31 healthy Caucasians in relation to the SLCO1B1 c.521T > C SNP. For clarity, some error bars have been omitted. Open squares indicate individuals with the c.521TT genotype (n = 16); solid squares indicate individuals with the c.521TC genotype (n = 11); solid triangles indicate individuals with the c.521CC genotype (n = 4). Data for the c.521TC group are calculated from 11 participants, because one of the original 12 participants was excluded as an outlier. This study shows that SLCO1B1 polymorphism has a marked effect on the plasma pharmacokinetics of active simvastatin acid, but not on those of the parent simvastatin lactone. The mean AUC and Cmax of simvastatin acid were about three-fold in participants with the SLCO1B1 c.521CC genotype as compared with those in participants with the c.521TT reference genotype. This finding supports the idea that simvastatin acid is a substrate of OATP1B1 and that it needs active transport in order to penetrate the hepatocyte plasma membrane. In contrast, the AUC and Cmax of the parent simvastatin lactone were only slightly and not statistically significantly different between participants with different SLCO1B1 genotypes. This indicates that simvastatin lactone either penetrates the hepatocyte plasma membrane via passive diffusion or that an uptake transporter other than OATP1B1 mediates its hepatic uptake. Molecular size, lipophilicity and charge are the major determinants of permeability of a lipid membrane to a drug. The molecular weight of simvastatin acid is 437 and Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. SLCO1B1 polymorphism and simvastatin Pasanen et al. 877 Pharmacokinetic variables of a single 40-mg oral dose of simvastatin in relation to SLCO1B1 polymorphism in 31 healthy Caucasians Table 2 SLCO1B1 genotype Simvastatin c.521TT (n = 16) c.521TC (n = 11)a c.521CC (n = 4) Simvastatin acid c.521TT (n = 16) c.521TC (n = 11)a c.521CC (n = 4) Cmax(ng/ml) 8.9 ± 4.7 10.2 ± 4.9 13.0 ± 3.7 2.2 ± 0.9 3.0 ± 1.2 6.6 ± 1.1c,d tmax(h) t1/2(h) AUC0–12(ng h/ml) AUC0–N(ng h/ml) 1.5 (0.5–2.0) 1.0 (0.5–5.0) 1.3 (0.5–2.0) 2.7 ± 0.7 3.2 ± 0.9 3.7 ± 0.2 24.7 ± 9.6 28.8 ± 14.4 34.5 ± 13.5 26.4 ± 10.5 31.9 ± 17.6 37.9 ± 14.7 5.0 (3.0–7.0) 4.0 (1.5–7.0)b 3.0 (2.0–5.0)b 3.3 ± 1.0 3.2 ± 1.1 3.4 ± 0.4 13.9 ± 5.3 17.4 ± 8.0 44.5 ± 7.4c,d 16.4 ± 6.4 20.1 ± 10.3 52.7 ± 12.5c,d Data are mean ± SD; tmax data are median (range). Cmax, peak plasma concentration; tmax, time to Cmax; t1/2, elimination half-life; AUC0–12, area under the plasma concentration–time curve from 0 to 12 h; AUC0–N, area under the plasma concentration–time curve from 0 h to infinity. a One of the 12 participants with the c.521TC genotype was excluded from the statistical analysis as an outlier. b P < 0.05 vs. participants with the c.521TT genotype. c P < 0.001 vs. participants with the c.521TT genotype. d P < 0.001 vs. participants with the c.521TC genotype. that of simvastatin lactone is 419. Simvastatin lactone is considerably more lipophilic than simvastatin acid, with experimentally determined octanol/water partition coefficients (logD7.0, at pH 7.0) of 4.40 for simvastatin lactone and 1.88 for simvastatin acid [35]. The pKa (dissociation constant) of simvastatin acid is 4.31 and that of simvastatin lactone is 13.49 (values obtained from the SciFinder Database, American Chemical Society). Thus, simvastatin acid is almost completely ionized (charged) in the human plasma with a pH of about 7.4, whereas the lactone is almost completely in the un-ionized form. As simvastatin acid is ionized in the plasma and because it is only mildly lipophilic, it poorly penetrates the hepatocyte plasma membrane via passive diffusion and thus requires active hepatic uptake. On the other hand, the more lipophilic and neutral simvastatin lactone may diffuse through the plasma membrane with relative ease. Similarly to simvastatin acid, the hydrophilic OATP1B1substrate pravastatin (logD7.0 – 0.47 [35], pKa 4.31) is almost completely ionized at the plasma pH and the SLCO1B1 c.521C allele is associated with markedly increased plasma pravastatin concentrations [26–28]. The highest Cmax and AUC of simvastatin acid were seen in one participant with the SLCO1B1 c.521TC genotype, who was clearly an outlier among the study participants (Fig. 2). The diplotype of that participant was *1A/*17. Two other participants also had this diplotype, but they had considerably lower simvastatin acid Cmax and AUC values. It is likely that characteristics other than SLCO1B1 genotype (e.g. genetic variability in carboxylesterase, CYP3A4, CYP2C8 or UDP-glucuronosyltransferase) also contributed to the high Cmax and AUC of simvastatin acid observed in this participant, but further studies beyond the scope of this investigation are required to determine the underlying causes. As all participants in the present study were non-expressers of CYP3A5, however, its polymorphism cannot explain the finding. The pattern of differences in the pharmacokinetics of simvastatin and simvastatin acid between participants with the c.521CC genotype and those with the c.521TT genotype is very similar to the changes observed during concomitant use of simvastatin with gemfibrozil. Gemfibrozil 1200 mg/day raised the AUC of simvastatin acid about 2.9-fold and that of simvastatin lactone about 1.4fold [33]. Gemfibrozil does not inhibit CYP3A4 [33], but it and its glucuronide conjugate inhibit CYP2C8 and OATP1B1 in vitro [36,37]. Considering the similarities in the effects of gemfibrozil and SLCO1B1 polymorphism on simvastatin pharmacokinetics, it appears that inhibition of the OATP1B1-mediated hepatic uptake of simvastatin acid could be the main mechanism of the pharmacokinetic interaction between gemfibrozil and simvastatin. Cyclosporine also markedly increases the plasma concentrations of simvastatin-derived inhibitors of HMG-CoA reductase [38]. Like gemfibrozil, cyclosporine is an inhibitor of OATP1B1 [20], but it also inhibits CYP3A4 and the P-glycoprotein efflux transporter [39,40]. In addition, cyclosporine inhibits the OATP1B3, OATP2B1 and the sodium/taurocholate cotransporting polypeptide transporters, which mediate the uptake of rosuvastatin in vitro [41]. No published data exist on the role of these transporters in the uptake of simvastatin or simvastatin acid. Concomitant use of simvastatin with gemfibrozil, cyclosporine or a potent CYP3A4 inhibitor is associated with an increased risk of myopathy and rhabdomyolysis [5–8]. OATP1B1-mediated uptake of statins into hepatocytes can be important in terms of enhancing their therapeutic efficacy and reducing their concentrations in peripheral blood [42]. Thus, reduced hepatic uptake of the active simvastatin acid in participants carrying the SLCO1B1 c.521C variant allele may result in both a reduced cholesterol-lowering efficacy and an increased risk of systemic adverse effects. In Japanese patients using pravastatin, atorvastatin or simvastatin, the SLCO1B1 c.521TC genotype was associated with impaired cholesterol-lowering efficacy as compared with the c.521TT genotype [43]. In addition, the effect of a single 40-mg dose of pravastatin on hepatic cholesterol synthesis was reduced in Caucasian carriers of the SLCO1B1*17 Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 878 Pharmacogenetics and Genomics 2006, Vol 16 No 12 haplotype containing c.521C [44]. Moreover, the SLCO1B1*15 haplotype, also containing c.521C, has been associated with myopathy induced by pravastatin or atorvastatin in Japanese patients [45]. The SLCO1B1 c.521T > C SNP is common in Caucasian and Asian populations, with a minor allele frequency of about 15–20% in Caucasians and 10–15% in Asians [32,46]. Therefore, SLCO1B1 polymorphism is likely to play a major role in interindividual variability in the pharmacokinetics of simvastatin, and possibly in its efficacy and toxicity, at the population level. Fig. 2 (a) 25 Cmax (ng/ml) 20 15 10 5 0 Lactone Acid c.521TT (b) Lactone Acid c.521TC Lactone Acid c.521CC 6 In conclusion, genetic polymorphism in SLCO1B1, encoding the hepatic uptake transporter OATP1B1, markedly affects the plasma concentrations of simvastatin acid, the active form of the HMG-CoA reductase inhibitor simvastatin. Genetic variability in OATP1B1 function can have clinically important consequences for the balance of risks and benefits of simvastatin treatment. 5 Acknowledgements We would like to thank Ms Eija Mäkinen-Pulli, Ms Lisbet Partanen, Ms Kerttu Mårtensson and Mr Jouko Laitila for skilful technical assistance. Half - life (h) 4 3 2 References 1 1 2 0 Lactone Acid c.521TT Lactone Acid c.521TC Lactone Acid c.521CC 3 (c) 100 4 AUC (ng h/ml) 90 80 5 70 6 60 7 50 8 40 30 9 20 10 0 Lactone Acid c.521TT 10 Lactone Acid c.521TC Lactone Acid c.521CC Individual Cmax (a), elimination t1/2 (b) and AUC0–N (c) values of simvastatin (lactone, solid circles) and simvastatin acid (acid, open circles) after a single 40-mg oral dose of simvastatin in 32 healthy Caucasians with different SLCO1B1 c.521T > C genotypes. The data of an outlier participant with the c.521TC genotype are shown with solid (simvastatin) and open (simvastatin acid) triangles. 11 12 13 Mauro VF. Clinical pharmacokinetics and practical applications of simvastatin. Clin Pharmacokinet 1993; 24:195–202. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383–1389. Vickers S, Duncan CA, Chen IW, Rosegay A, Duggan DE. Metabolic disposition studies on simvastatin, a cholesterol-lowering prodrug. Drug Metab Dispos 1990; 18:138–145. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990; 343:425–430. Rosenson RS. Current overview of statin-induced myopathy. Am J Med 2004; 116:408–416. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690. Williams D, Feely J. Pharmacokinetic–pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet 2002; 41: 343–370. Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipidlowering drugs. JAMA 2004; 292:2585–2590. Prueksaritanont T, Gorham LM, Ma B, Liu L, Yu X, Zhao JJ, et al. In vitro metabolism of simvastatin in humans [SBT]: identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos 1997; 25:1191–1199. Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol 2003; 56:120–124. Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, et al. Glucuronidation of statins in animals and humans: a novel mechanism of statin lactonization. Drug Metab Dispos 2002; 30:505–512. Neuvonen PJ, Kantola T, Kivistö KT. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin Pharmacol Ther 1998; 63:332–341. Kantola T, Kivistö KT, Neuvonen PJ. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther 1998; 64:177–182. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. SLCO1B1 polymorphism and simvastatin Pasanen et al. 879 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice-simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther 1998; 64:477–483. Kivistö KT, Niemi M, Schaeffeler E, Pitkälä K, Tilvis R, Fromm MF, et al. Lipid-lowering response to statins is affected by CYP3A5 polymorphism. Pharmacogenetics 2004; 14:523–525. Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 1999; 274:17159–17163. Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, et al. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem 1999; 274:37161–37168. Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 2004; 447:653–665. Nakai D, Nakagomi R, Furuta Y, Tokui T, Abe T, Ikeda T, et al. Human liver-specific organic anion transporter, LST-1, mediates uptake of pravastatin by human hepatocytes. J Pharmacol Exp Ther 2001; 297: 861–867. Shitara Y, Itoh T, Sato H, Li AP, Sugiyama Y. Inhibition of transportermediated hepatic uptake as a mechanism for drug–drug interaction between cerivastatin and cyclosporin A. J Pharmacol Exp Ther 2003; 304: 610–616. Simonson SG, Raza A, Martin PD, Mitchell PD, Jarcho JA, Brown CD, et al. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther 2004; 76:167–177. Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther 2004; 311:139–146. Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15 + C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genom 2005; 15:513–522. Kopplow K, Letschert K, König J, Walter B, Keppler D. Human hepatobiliary transport of organic anions analyzed by quadruple-transfected cells. Mol Pharmacol 2005; 68:1031–1038. Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem 2001; 276:35669–35675. Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther 2003; 73:554–565. Mwinyi J, Johne A, Bauer S, Roots I, Gerloff T. Evidence for inverse effects of OATP-C (SLC21A6) 5 and 1b haplotypes on pravastatin kinetics. Clin Pharmacol Ther 2004; 75:415–421. Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1). Pharmacogenetics 2004; 14: 429–440. Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther 2005; 78:330–341. Chung JY, Cho JY, Yu KS, Kim JR, Oh DS, Jung HR, et al. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther 2005; 78: 342–350. 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 Chen C, Mireles RJ, Campbell SD, Lin J, Mills JB, Xu JJ, et al. Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos 2005; 33: 537–546. Pasanen MK, Backman JT, Neuvonen PJ, Niemi M. Frequencies of single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide 1B1 SLCO1B1 gene in a Finnish population. Eur J Clin Pharmacol 2006; 62:463–472. Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther 2000; 68:122–129. Wu Y, Zhao J, Henion J, Korfmacher WA, Lapiguera AP, Lin CC. Microsample determination of lovastatin and its hydroxy acid metabolite in mouse and rat plasma by liquid chromatography/ion spray tandem mass spectrometry. J Mass Spectrom 1997; 32:379–387. Ishigami M, Honda T, Takasaki W, Ikeda T, Komai T, Ito K, et al. A comparison of the effects of 3-hydroxy-3-methylglutaryl-coenzyme a (HMG-CoA) reductase inhibitors on the CYP3A4-dependent oxidation of mexazolam in vitro. Drug Metab Dispos 2001; 29:282–288. Shitara Y, Hirano M, Sato H, Sugiyama Y. Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/ OATP1B1:SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin: analysis of the mechanism of the clinically relevant drug–drug interaction between cerivastatin and gemfibrozil. J Pharmacol Exp Ther 2004; 311:228–236. Yamazaki M, Li B, Louie SW, Pudvah NT, Stocco R, Wong W, et al. Effects of fibrates on human organic anion-transporting polypeptide 1B1-, multidrug resistance protein 2- and P-glycoprotein-mediated transport. Xenobiotica 2005; 35:737–753. Arnadottir M, Eriksson LO, Thysell H, Karkas JD. Plasma concentration profiles of simvastatin 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitory activity in kidney transplant recipients with and without ciclosporin. Nephron 1993; 65:410–413. Jacobsen W, Kirchner G, Hallensleben K, Mancinelli L, Deters M, Hackbarth I, et al. Comparison of cytochrome P-450-dependent metabolism and drug interactions of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors lovastatin and pravastatin in the liver. Drug Metab Dispos 1999; 27:173–179. Stapf V, Thalhammer T, Huber-Huber R, Felberbauer F, Gajdzik L, Graf J. Inhibition of rhodamine 123 secretion by cyclosporin A as a model of P-glycoprotein mediated transport in liver. Anticancer Res 1994; 14: 581–585. Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology 2006; 130:1793–1806. Kim RB. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) and genetic variability (single nucleotide polymorphisms) in a hepatic drug uptake transporter: what’s it all about? Clin Pharmacol Ther 2004; 75:381–385. Tachibana-Iimori R, Tabara Y, Kusuhara H, Kohara K, Kawamoto R, Nakura J, et al. Effect of genetic polymorphism of OATP-C (SLCO1B1) on lipidlowering response to HMG-CoA reductase inhibitors. Drug Metab Pharmacokinet 2004; 19:375–380. Niemi M, Neuvonen PJ, Hofmann U, Backman JT, Schwab M, Lütjohann D, et al. Acute effects of pravastatin on cholesterol synthesis are associated with SLCO1B1 (encoding OATP1B1) haplotype *17. Pharmacogenet Genom 2005; 15:303–309. Morimoto K, Ueda S, Seki N, Igawa Y, Kameyama Y, Shimizu A, et al. OATP-C (OATP1B1)*15 is associated with statin-induced myopathy in hypercholesterolemic patients [Abstract]. Clin Pharmacol Ther 2005; 77:P21. Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, et al. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther 2002; 302:804–813. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.