* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Management of VSD (Ventricular septal defect)
Survey
Document related concepts
Coronary artery disease wikipedia , lookup
Artificial heart valve wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Aortic stenosis wikipedia , lookup
Congenital heart defect wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
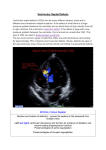
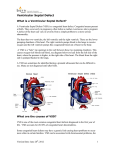
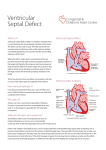
Management of VSD (Ventricular septal defect) Overview The most common lesion seen in congenital heart disease (about 2.5 per 1000 live births). Ventricular defects may be located anywhere in the ventricular septum, single or multiple, of variable size and shape, with associated defects. Ventricular defects are classified by their location in the septum -> 4 types. Anatomy Anatomy 1. Membranous defects: Originally considered the most common type, are now just more common in complicated lesions than muscular defects. A small translucent structure located immediately superior to the division of the septal band and adjacent to the commissure between the anterior and septal leaflets of the tricuspid valve. It lies directly under the aortic valve on the left side and overlaps a small segment of the right atrium. Associated aortic valvar abnormalities, ventricular Aortic rim Perimembranous VSD Anatomy 2. Muscular defects: May be located anywhere in the apical, mid, anterior or posterior muscular septum and are often multiple. With echocardiography, now more frequently identified & more common than membranous defects when uncomplicated. Anatomy 3. Infundibular (subpulmonary) defects: Located under the pulmonary valve when viewed from the right ventricle & immediately beneath the aortic valve viewed from the left ventricle. Associated prolapse of the adjacent right coronary aortic valve cusp… Anatomy 4. Endocardial cushion type of defects: Located beneath the tricuspid valve, extending to the tricuspid valve ring. Occupy the area when an atrioventricularis communis opening would be found. MANAGEMENT Management 1. Influence of size of defect: a) Small VSDs: The child who has reached the age of 6 months without evidence of congestive heart failure and pulmonary hypertension can be managed conservatively. After the 1st year of life, asymptomatic infants known to have small, persistent patent defects should be examined every 3 years or so to watch for aortic valve prolapse or regurgitation, document any defect decrease in size. Prophylactic antibiotics to prevent infective endocarditis. Management b) Large VSDs: Mortality rate with surgical closure < 1 % repair even in the first few months of life. Neonates & infants with poor growth, an single unrestrictive defect, pulmonary artery pressure (PAP) at or near systemic level: surgical closure should be undertaken. Those with multiple defects, as long as they are reachable through the tricuspid valve: also should undergo surgical closure. Management Babies with restrictive defects, PAP < 60 % systemic level, adequate growth: can be followed medically. In older patients, large defects with significant shunts & left ventricular enlargement: should be closed either surgically or catheterdelivered devices. Catheter-delivered devices have been mostly applied to patients > 1 year old. Management 2. Influence of type of defect: a) Membranous defects: Many cases often become smaller, with up to 27 %, including some large defects, closing spontaneously -> patients with good growth & low PAP should be followed medically. Malalignment membranous defects are rare, usually large & associated with pulmonary hypertension: should be surgically closed. Management b) Muscular defects: Very common, often multiple. Lesions may close spontaneously follow infants conservatively for some months as long as they are doing well. If significant shunting persists with elevated PAP: primary closure indicated. Apical and anterior defects are best managed with catheter-delivered devices (in patients > 6 months), whereas those proximal to the moderator band are accessible surgically at any age. Pulmonary artery banding is essentially no longer A. At age 4 months, huge left-to-right shunt (Qp/Qs > 4/1) through multiple muscular VSDs (arrows) B. At age 14 months, spontaneous closure of defects with elimination of shunt. Management c) Infundibular (Subpulmonary) defects: Rarely small, not known to get smaller, aortic regurgitation develops commonly ALL patients with significant anatomic defects are referred to surgeons after the age of 6 months or earlier if there is growth failure. d) Endocardial cushion type of defect: Uncommonly small, do not regress spontaneously or get smaller surgery is ALMOST invariably needed. COURSE 1. Spontaneous diminution in size: Frequencies vary considerably, related to age, defect location, follow-up duration, methods of detection, particularly echocardiography. Inlet (endocardial cushion), typical malalignment & subpulmonary defects are usually large and remain so. Membranous and muscular defects often decrease or close. COURSE 2. Development of Pulmonary Vascular Disease (Eisenmenger’s complex): The development of permanent Pulmonary Vascular Disease is very rare before the first birthday. More common with a large VSD, multiple VSDs or associated patent ductus arteriosus (PDA). Early surgery can minimalize this disease. Development of Pulmonary Vascular Disease (Eisenmenger’s complex) Any susgestion of pulmonary hypertension in patients > 12 months with VSD require cardiac catheterization for evaluation. Virtually all children with VSD & any evidence of pulmonary vascular disease undergo surgical correction in the first year of life because it is highly likely that pulmonary vascular disease must be of recent onset. Development of Pulmonary Vascular Disease (Eisenmenger’s complex) Beyond the age of 12 months, the decision to operate depends on whether the vascular change is minimal or advanced, is of recent onset or show evidence of reversibility. Reference for VSD closure: pulmonary resistance estimated < 8 U/m2 & any evidence of developing pulmonary vascular disease, Qp/Qs > 2:1, clearly response to dilator therapy (Oxygen, Nitric oxide). Development of Pulmonary Vascular Disease (Eisenmenger’s complex) Contraindication for VSD closure: pulmonary resistance > 8 U/m2, particularly those who have no left-to-right shunt, those older than 2 years, and those who show no response to pulmonary arterial dilatation. Women with pulmonary vascular disease secondary to congenital heart problems must not be pregnant & not use pills or mechanical devices of contraception. COURSE 3. Acquired aortic regurgitation: Due to prolapse of one or more valve cusps into the adjacent ventricular defect (membranous or subpulmonary), a bicuspid aortic valve… Acquired aortic regurgitation a) Subpulmonary defects with aortic regurgitation: It is reasonable to recommend surgical closure of any subpulmonary defects (other than rare tiny ones) shortly after discovery (Stable patient > 15 years old, minimal shunt, mild regurgitation may be continued medical observation). Mild regurgitation: closure alone is sufficient. Moderate or more regurgitation: valve plasty is quite effective. Valve replacement is rarely needed, except in older patients. Acquired aortic regurgitation b) Membranous defect with aortic regurgitation: Obvious prolapse, and a tricuspid valve: similar management indications as for subpulmonary defects are reasonable. Note: Strict endocarditis prophylaxis is vital in these patients, before & after surgery. COURSE 4. VSDs & aortic stenosis: VSDs may be membranous or muscular, aortic stenosis may be valvar or subvalvar. VSDs may decrease in size or close spontaneously and aortic stenosis lesions may progress -> management varies considerably & depends on the current status. VSDs & aortic stenosis Stable small defects & mild or less obstruction can be followed medically indefinitely. Stable neonates with moderate septal defects & minimal obstruction can also be followed medically, initially frequently. VSDs & aortic stenosis Symptomatic, large defect with high PAP, mild stenosis: surgical closure alone is necessary. Small defect with significant valvar outflow obstruction: ballon dilation is the treatment of choice. Moderate or more subaortic obstruction: surgical reresection is necessary but better delayed ≥ 10 years because of common recurrent obstruction. Note: continued endocarditis prophylaxis is essential. COURSE 5. VSD & secundum atrial septal defect (ASD): Some ASDs are dilated patent foramen ovale (PFO) because of the left atrial hypertension & atrial left-to-right shunting which disappear after closure of VSD. In patients with true secundum ASD, the left atrial overload resulting from the VSD is relieved by the ASD, and there is no atrial hypertension. This distinction is not important because it has the same operation route. COURSE 6. VSD & PDA: When both defects are large and the amount of left-to-right shunting is determined by the pulmonary resistance, even if it is large, may not affect the size of shunt. From a practical standpoint, both defects are closed at surgery in the infant without any additional risk. VSD & PDA Restrictive VSD: PDA closure alone may be benificial. Significant lesions in very young patients: surgical closure of both defects. Normally growing infant with clearly restrictive defects may be followed medically; if PDA persists, it may be closed by coiloccluded catheterization. VSD & PDA Older patient with significant lesions and low pulmonary resistance: close defects surgically or by devices. Elevated pulmonary resistance suspected: catheterization to evaluate response to oxygen & nitric oxide and temporary occlusion and device closure of the ductus if indicated. COURSE 7. VSDs with Pulmonary stenosis: a) Acyanotic Tetralogy of Fallot: differential diagnosis by large VSD, infundibular pulmonary stenosis, overriding aorta, the systemic level right ventricular pressure. b) Ventricular defect and valvar pulmonary stenosis: Treatment is indicated or not depends on the severity of the defects. c) VSD with double-chambered right ventricle: Muscle bundles traverse and obstruct the right ventricular outflow tract, lesion is usually COURSE 8. VSD & Mitral valve (MV) disease: a) VSD & Mitral valve stenosis: Minimal MV stenosis, moderate or less pulmonary hypertension: management is the same as that for the usual VSD. Moderate MV stenosis, VSD of some size, there will be systemic levels of pressure in the pulmonary artery. The amount of left-to-right shunting depends largely on the comparative levels of pulmonary & systemic resistance. VSD & Mitral valve stenosis Severe MV stenosis, level of pulmonary resistance may become so high -> ventricular shunting reverses, cyanosis. The ultimate success of management depends on the success in treating MV stenosis. Infant with severe MV stenosis, large VSD: surgical defect closure + valvuloplasty. If VSD is small: ballon dilation may apply to MV stenosis. VSD & Mitral valve stenosis Older patient with large VSD, significant MV stenosis: Catheterization for hemodynamic evaluation. If there is high pulmonary resistance, ballon dilation may relieve stenosis, decrease resistance & increase left-to-right shunting to close VSD safely. COURSE b) VSD & mitral regurgitation: management depends on clinical status, degree of regurgitation, size of VSD. Both lesions are mild: medical management indicated. Large left-to-right shunt at the ventricular level & not severe regurgitation: VSD closure alone may improve the regurgitation. Severe regurgitation: valvuloplasty is usually effective in the short term. Catheterization data in a 4-months-old infant with 2:1 left-to-right shunt, membranous VSD (black arrow), and moderate mitral regurgitation through anterior leaflet cleft (open arrow). Four years later, the VSD had spontaneously closed, and at age 10 years, the mitral cleft was successfully closed surgically. SUMMARY Type Size AP/PS RP/RS Qp/Qs Ia Resistive <0.3 <0.3 1.0-1.5 Ib Resistive <0.3 <0.3 1.5-2 IIa Resistive 0.3-0.7 <0.5 >2 IIb Non resistive 0.7-1 >2 <0.8 Hemodynamic Classification Nom III VI Size Non resistive Non resistive AP/PS RP/RS Qp/Qs >1 >1 <1 <0.7 <0.5 >2 VSD Ia and Ib: Malade de Roger VSD IIa and IIb: VSD high debit VSD III: Eissenmenger (large VSD) VSD IV: VSD and Pulmonary stenosis VSD management VSD IA (Roger):Follow up VSD IIa: Surgery if Medical treatment failure Pulmonary pressure increasing LV too large More than 1 year VSD IIb: Surgery if single VSD, PA banding multiple VSD patient <9month total correction after VSD+IA, VSD + RVOT obstruction…: Surgery VSD type IB ???? + 75% of VSD + Wait and see ? + Osler, AR, LV hemodynamic … + Surgery complication? + Intervention complication? Reference 1. 2. 3. 4. 5. 6. 7. 8. 9. Lillehei C.W., Cohen M., Warden H.E., Ziegler N.R., Varco R.L. The results of direct vision closure of ventricular septal defects in eight patients by means of controlled cross circulation. Surg Gynecol Obstet 1955;101:447-466. Kumar K., Lock J.E., Geva T. Apical muscular ventricular septal defects between the left ventricle and the right ventricular infundibulum. Diagnostic and interventional considerations. Circulation 1997;95:12071213.[Abstract/Free Full Text] Jonas R.A. Cerebral protection in infants. In: Yacoub M., Pepper J., eds. Annual of cardiac surgery, 8th ed. London: Current Science, 1994:153-160. Cooley D.A., Belmonte B.A., DeBakey M.E., Latson J.R. Temporary extracorporeal circulation in the surgical treatment of cardiac and aortic disease. Report of 98 cases. Ann Surg 1957;145:898-914. Doty D.B., McGoon D.C. Closure of perimembranous ventricular septal defect. J Thorac Cardiovasc Surg 1983;85:781-790.[Medline] Stellin G., Milanesi O., Rubino M., et al. Repair of tetralogy of Fallot in the first six months of life. Ann Thorac Surg 1995;60:S588-S591. Hobbins S.M., Izukawa T., Radford D.J., Williams W.G., Trusler G.A. Conduction disturbances after surgical correction of ventricular septal defect by the atrial approach. Br Heart J 1979;41:289293.[Abstract/Free Full Text] Nadal text book of Pediatric cardiology. 2009 Anderson R.H., Wilcox B.R. The surgical anatomy of ventricular septal defect. J Card Surg 1992;7:17-35.[Medline] 1. 2. 3. 4. 5. 6. 7. 8. 9. Pridjian A.K., Pearce F.B., Culpepper W.S., Williams L.C., Van Meter C.H., Ochsner J.L. Atrioventricular valve competence after takedown to improve exposure during ventricular septal defect repair. J Thorac Cardiovasc Surg 1993;106:1122-1125.[Abstract] Griffiths S.P., Turk G.K., Ellis K., et al. Muscular ventricular septal defects repaired with left ventriculotomy. Am J Cardiol 1981;48:877-886.[Medline] Von Segesser L.K., Fasnacht M.S., Vogt P.R., Genoni M., Arbenz U., Turina M.I. Prevention of residual ventricular septal defects with fibrin sealant. Ann Thorac Surg 1995;60:511-516.[Abstract/Free Full Text] Fishberger S.B., Bridges N.D., Keane J.F., et al. Intraoperative device closure of ventricular septal defects. Circulation 1993;88:205-209. Breckenridge I.M., Stark J., Waterston D.J., Bonham-Carter R.E. Multiple ventricular septal defects. Ann Thorac Surg 1972;13:128-136.[Medline] Momma K., Toyama K., Takao A., et al. Natural history of subarterial infundibular ventricular septal defect. Am Heart J 1984;108:1312-1317.[Medline] Chauvaud S., Serraf A., Mihaileanu S., et al. Ventricular septal defect associated with aortic valve incompetence. Ann Thorac Surg 1990;49:875-880.[Abstract] Yacoub M.H., Khan H., Stavri G., Shinebourne E., Radley-Smith R. Anatomic correction of the syndrome of prolapsing right coronary aortic cusp, dilatation of the sinus of Valsalva and ventricular septal defect. J Thorac Cardiovasc Surg 1997;113:253261.[Abstract/Free Full Text] Kirklin J.W. The movement of cardiac surgery to the very young. In: Crupi G., Parenzan L., Anderson R.H., eds. . Perspectives in pediatric cardiology. Mt Kisco, New York: Futura Publishing Co, 1989:3-20.