* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download High-resolution retinal microscopy using MEMS
Ultrafast laser spectroscopy wikipedia , lookup
Imagery analysis wikipedia , lookup
Surface plasmon resonance microscopy wikipedia , lookup
Optical tweezers wikipedia , lookup
Reflector sight wikipedia , lookup
Retroreflector wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Photon scanning microscopy wikipedia , lookup
Hyperspectral imaging wikipedia , lookup
Scanning SQUID microscope wikipedia , lookup
3D optical data storage wikipedia , lookup
Chemical imaging wikipedia , lookup
Preclinical imaging wikipedia , lookup
Super-resolution microscopy wikipedia , lookup
Optical coherence tomography wikipedia , lookup
Nonimaging optics wikipedia , lookup
Confocal microscopy wikipedia , lookup
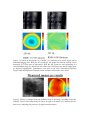
High-resolution retinal microscopy using MEMS-based adaptive optics Yaopeng Zhou and Thomas Bifano ([email protected]) College of Engineering, Boston University The availability of precisely controllable micromachined deformable mirrors (µDMs) made using microelectromechanical (MEMS) systems technology has enabled rapid development of adaptive optics in several new applications. In this paper, the design and testing of a retinal imaging system is detailed, with an emphasis on the adaptive optical components and their performance. Retinal imaging is a major diagnostic modality for retinal disease, and can play a critical role for diagnosing systemic diseases such as diabetes and eye-specific diseases such as macular degeneration and diabetic retinopathy, the leading causes of blindness. It is demonstrated in this work that the µDM can enable diffraction-limited imaging of microscale features on and below the surface of the retina, through real-time compensation of aberrations introduced by the eye's cornea and lens. The scanning laser ophthalmoscope, invented in 1980 by Rob Webb, allows highcontrast, high resolution imaging of the retina. Its confocal detector greatly improves contrast and allows sub-surface imaging, permitting detailed study of the vasculature that supplies capillary blood flow to the retina. A conventional scanning laser ophthalmoscope is limited to resolution of ~8-20 µm laterally and 250-300 µm axially. This resolution is about a factor of five worse than the theoretical resolution of the instrument imposed by its diffraction limited performance. The difference between 10µm resolution (typical SLO performance) and 2µm resolution (diffraction-limited SLO performance) is important because it distinguishes between an instrument that can resolve capillary blood flow and one that can not. Both major causes of blindness worldwide are directly related to physiological defects in capillary blood flow. An instrument that could achieve diffraction limited resolution (particularly on animal subjects) might enable significant progress in the study, diagnosis, and treatment of these diseases. Moreover, direct observation of capillary blood flow in the retina would provide a valuable tool for the study of systemic diseases related to vascular failure – especially diabetes. To achieve diffraction-limited performance in an SLO, it is necessary to compensate for aberrations of the eye, which account for nearly all degradation in retinal image quality. It is a direct result of the eye's poor performance as an objective lens that the resolution of an SLO is inadequate for resolving micrometer-scale cellular details. Adaptive optics (AO) scanning confocal microscopy systems, by using a deformable mirror and real time control system to correct the aberrations of the eye, can improve resolution in retinal imaging. In the project reported in this paper, an adaptive optics scanning laser ophthalmoscope for imaging of mouse retinas is described. Figure 1 is a block diagram of the optical system. The µDM is used as a wavefront correction element for AO system. The wavefront is measured using a Shark-Hartmann wavefront sensor. This sensor provides 244 discrete measurements of wavefront slope, distributed uniformly across the aperture (at each of 121 points in an 11 x 11 grid, slope is measured in two orthogonal directions). The deformable mirror is located at an optical plane conjugate to the Shack-Hartmann lenslet array, and provides 140 degrees of freedom for adjusting wavefront shape (actuators are oriented in a 12 x 12 grid pattern, without the four corner elements). The relay optics are designed to locate the Shack Hartman lenslet centers interstitially with respect to the µDM actuator centers, although this alignment is not critical. Figure 1: Adaptive optics scanning laser ophthalmoscope test bed schematic. A collimated laser beam is focused by the mouse eye to a spot on the retina. A scanning subsystem forces the spot to raster across a small area at or below the retinal surface. Light scattered from the spot passes back through the scanner (e.g. is de-scanned), and then reflects from the µDM, which is located at a plane conjugate to the mouse pupil. The wavefront is relayed to a Shack-Hartmann wavefront sensor that measures the effects of aberrations on the wavefront. Feedback from this sensor is used in closed loop control to adjust the shape of the µDM to compensate for aberrations of the eye. The corrected beam is relayed to a confocal detector consisting of a precisely aligned aperture and a fast photodetector. The output of the photodetector, when synchronized with the scanner, produces a diffraction-limited video image of the scanned area of the retina. Because the Shack-Hartmann sensor measures 244 distributed slopes and the deformable mirror provides 140 distributed displacements, a multi-input multi-output control scheme is needed. We elected to implement a reconstructor based on modal control. Each actuator was perturbed independently, and the effect on the wavefront sensor was observed. Using this data and assuming system linearity, a reconstructor matrix was built to map any matrix of actuator deflections to a unique sensor matrix. This reconstructor was inverted using a singular value decomposition to provide a map from any sensor matrix to a best fit actuator deflection matrix. This constitutes a calibration step that is necessary only once, before beginning closed-loop control. Subsequently, control is achieved by multiplying measured sensor data by the inverted reconstructor matrix to estimate wavefront error, and then using this error signal in an integral control loop to adjust the µDM. Frequently, optical wavefront data is represented by Zernike decomposition, in which the surface is represented by a series of ortho-normal polynomials defined on a unit circle. Coefficients of Zernike polynomials provide a widely recognized basis for evaluating wavefront aberrations. The first three Zernike terms correspond to piston and tip and tilt of the wavefront: the only three terms that do not affect image resolution. The next three terms correspond to familiar optical aberrations (astigmatism and defocus). Higher order aberrations correspond to less familiar aberrations, including coma, trefoil, spherical aberration, and many more that are unnamed. A model eye without aberrations was introduced into the optical system described in Figure 1, and the closed-loop controller was commanded to produce, sequentially, wavefront shapes corresponding to each of the first twenty one Zernike polynomials (exclusive of piston, tip, and tilt). The amplitude of the desired shape was fixed at 1.0µm. Figure 2 shows the result, which confirms the capability of the µDM to control wavefront shape. Figure 2: Measured wavefront for Zernike modes 4-21, as obtained by closed-loop control with the µDM in the adaptive optics scanning laser ophthalmoscope. Subsequently a reticle grid was positioned at the retinal plane of the model eye, and the eye was adjusted to introduce aberration through defocus. The confocal scanned image was recorded before and after adaptive optical compensation using the closed-loop controller, along with the measured wavefront data. Results are shown in Figure 3. Finally, a mouse was introduced into the apparatus. Biological imaging was significantly more challenging than model-eye imaging, due in part to anesthesia-related cataracts forming in the mouse, tear-film reflections, and excessive scattering from anterior retinal surfaces. Nevertheless, images were obtained and an example is shown in Figure 4, along with a wavefront map. These images were obtained without AO. Figure 3: Control of aberrations in a "model" eye consisting of a reticle target and an aberrated imaging lens. With the AO system off, the image was blurred, and the sensor measured 0.2727nm (rms) of aberration. With the AO system on and controlling in a closed feedback loop, the aberration was reduced to 0.0815µm (rms) and the image quality improved significantly. Quantitative tests with an Air Force Resolution Reference Target confirmed diffraction-limited system resolution while under AO control. Figure 5: Mouse eye imaged using the AOLSO. At left is the image, including clearly discernable blood vessels measuring 10-20µm. At right is the mouse eye's measured wavefront error, indicating the presence of significant aberrations.