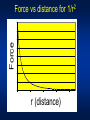
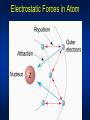
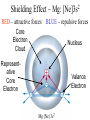
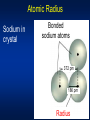
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Periodic Table
Survey
Document related concepts
Transcript
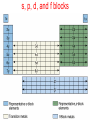
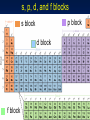
Chapter 6 Periodic Table & Periodic Law 6.1 Development of the Modern Periodic Table 6.2 Classification of the Elements 6.3 Periodic Trends Section 6.1 Development of the Modern Periodic Table The periodic table evolved over time as scientists discovered more useful ways to compare and organize the elements. • Trace the development of the periodic table from the law of octaves through the current table ordered by atomic number, including the scientists who contributed to each stage of development. • State the periodic law. • Identify key features of the periodic table. • Explain the common feature of elements within a group. Section 6.1 Development of the Modern Periodic Table • Identify the portion of the periodic table the terms “representative elements”, “transition metals”, “inner transition metals”, “alkali metals”, “alkaline earth metals”, metalloids, halogens, “noble gases”, “lanthanide series”, “actinide series”, and “transuranium elements” refer to and be able to give examples of some characteristics of the elements found in these regions. • State the number of naturally occurring elements on Earth, the total number of elements that are currently formally recognized as existing, and the names of the 2 most recently recognized elements. Section 6.1 Development of the Modern Periodic Table • State the typical characteristics of metals, nonmetals and metalloids and be able to give an example of an element in each of these categories • Identify the states and colors of the 4 halogens at room temperature and describe the trend in reactivity among the halogens. • Identify what is different about copper, gold, and mercury compared with other transition metals. Section 6.1 Development of the Modern Periodic Table Key Concepts • The elements were first organized by increasing atomic mass, which led to inconsistencies. Later, they were organized by increasing atomic number. • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same group. Section 6.1 Development of the Modern Periodic Table Key Concepts • Elements are classified as either metals, nonmetals, or metalloids. History of Development John Newlands (~ mid 1860s) • Octave rule for elements ordered by atomic mass History of Development Lothar Meyer, Dimitri Mendeleev • Both demonstrated relationships between elemental properties and atomic mass • Mendeleev given more credit for idea • Published first table in 1872 Dimitri Mendeleev First Periodic Table • Ordered by atomic mass Predicted existence/properties of undiscovered elements • Scandium (Sc) • Gallium (Ga) • Germanium (Ge) Prediction of Germanium Properties Property Atomic Mass Density, g/cm3 Color Dens. Oxide BP of chloride Dens. Chloride Predicted Eka-Silicon (1871) 72 5.5 Dirty gray EsO2: 4.7 EsCl4: < 100 C EsCl4: 1.9 Observed Germanium (1886) 72.6 5.47 Grayish white GeO2: 4.703 GeCl4: 86 C GeCl4: 1.887 Henry Moseley (~1913) Known that some elements in wrong order Used term atomic number (AN) to indicate amount of charge in nucleus (determined this charge from positions of spectral lines) 6 years prior to proton discovery Arrangement by AN fixed periodic table problems Contributions to Classification of the Elements (Table 6.2) positive charge Periodic Law There is a periodic repetition of chemical and physical properties of the elements when they are arranged by increasing atomic number Elements – Periodic Table Ordered by atomic number (number of protons in nucleus) Columns (vertical) = groups or families Current IUPAC numbering system is 1-18 Elements in same group tend to have similar chemical and physical properties Rows (horizontal) = periods (currently 7) Table “periodic” because pattern of variation of chemical and physical properties repeats in each period Elements - Newest Elements with atomic numbers 114 and 116 officially named 5/2012 as Flerovium (Fl) and Livermorium (Lv), respectively. Copernicium (Cn), atomic number 112 officially named 2/2010 Elements 113, 115, 117 (newest – April 2010), 118 have claimed to have been made, but evidence not yet convincing enough for official recognition by IUPAC Official current total = 114 elements Periodic Table of Elements Each box shows atomic number and the element’s symbol Newest elements Fl & Lv; remainder claimed to have been made but have not been officially recognized as existing Periodic Table – Fig. 6.5 Representative Elements Modern Periodic Table Representative Elements • Groups 1-2, 13-18 • Wide range of chemical & physical properties Transition Metals • Groups 3 to 12 General Classifications Metals Nonmetals Metalloids Classifications Metals • Shiny when smooth & clean • Solid at room temperature • Good conductors of heat & electricity • Ductile • Malleable Metal Classifications Alkali Metals – Group 1 Li, Na, K, Rb, Cs, Fr Very reactive, soft, mostly exist as compounds with other elements Metal Classifications Alkaline Earth Metals – Group 2 Be, Mg, Ca, Sr, Ba, Ra Also reactive, but not as much as the alkali metals Ca & Mg important nutrients (as ions, not as elements) Mg alloys useful as light-weight materials (bikes, laptop cases) Transition and Inner Transition Metals Inner transition metals: Lanthanide & Actinide Series Metal Classifications Transition Metals / Inner Transition Metals Groups 3 to 12 Inner transition metals consist of two parts • Lanthanide series - Ce through Lu • Actinide series – Th through Lr • All elements past U (AN 92) are synthetic (man made) Called transuranim elements Some Transition Metals Only transition metals not having a silver/gray color Cr An Atypical Transition Metal Mercury Only transition metal that is liquid at room temperature Metalloids Semi-Metals / Metalloids The “staircase” – dividing line • Steps start between boron and aluminum • Elements on either side of the dividing line are metalloids except for Al • Some texts do not include Po • Most do not include At – highly radioactive, estimated that total amount in Earth’s crust <30 g at any time – hard to study Silicon & germanium most important Nonmetals Nonmetals Gases or brittle, dull-looking solids Poor conductors of heat and electricity Group 16 Nonmetals O, S, Se, Te Sulfur Selenium Group 17 (Halogens): F, Cl, Br, I All diatomic (F2, etc) States @ RT: F2(g), Cl2(g), Br2(l), I2(s) Colors: colorless, pale greeen, dark redbrown, very dark violet (almost black) All reactive, but F2 most, I2 least Group 18 - Noble Gases He, Ne, Ar, Kr, Rn Unreactive Used in lasers, light bulbs, signs and certain types of welding (TIG, argon) Chapter 6 Periodic Table & Periodic Law 6.1 Development of the Modern Periodic Table 6.2 Classification of the Elements 6.3 Periodic Trends Section 6.2 Classification of the Elements Elements are organized into different blocks in the periodic table according to their electron configurations. • Explain why elements in the same group have similar properties. • Identify the four blocks of the periodic table based on their electron configuration. • Identify the two exceptions in normal orbital filling order in the period 4 transition metals. Section 6.2 Classification of the Elements Key Concepts • The periodic table has four blocks (s, p, d, f). • Elements within a group have similar chemical properties. • The group number for elements in groups 1 and 2 equals the element’s number of valence electrons. • The energy level of an atom’s valence electrons equals its period number. Periodic Table Organization Basic organization of periodic table by Mendeleev (~1872) was by recurring trends in elemental properties Electron not discovered until 1897 Schrodinger model atom was ~1927 Agreement between property based table and electron configuration based table demonstrates influence of electron configuration on properties Organizing Elements by Electron Configuration Group 1 – see table 6.3 Peri Elem od ent Electron Configuration 1 H 1s1 2 Li 1s22s1 3 Na 1s22s22p63s1 4 K 1s22s22p63s23p64s1 N. Gas Config 1s1 [He]2s1 [Ne]3s1 [Ar]4s1 Organizing Elements by Electron Configuration Group 1 – see table 6.3 Peri Element N. Gas # Valence od Configuration Electrons 1 H 1s1 1 2 Li [He]2s1 1 3 Na [Ne]3s1 1 4 K [Ar]4s1 1 Organizing Elements by Electron Configuration Elements in a group have similar chemical properties because they have the same number of valence electrons • Group 1 • Group 2 • Group 13 • Group 14 ns1 ns2 ns2np1 ns2np2 1 2 3 4 Organizing Elements by Electron Configuration # of valence electrons = # of electrons in highest principal energy level = group number (groups 1 and 2) = group number – 10 (groups 13 to 18) He (18) exception (2 valence electrons) For transition metals, # valence electrons can vary and may not be simply related to the group number Organizing Elements by Electron Configuration By definition, energy level of an element’s valence electrons = period of element Ga [Ar]3d104s24p1 period 4 s-, p-, d-, and f- Block Elements Block: section of the periodic table that corresponds to the energy sublevel being filled with valence electrons • s, p, d, and f sublevels s-block elements Groups 1 and 2 • 1 s1 • 2 s2 Can only have 2 s-block groups because s sublevel only holds 2 electrons p-block elements Groups 13 to 18 (except He) • • • • • • • s sublevel is already filled (s2) 13 p1 14 p2 15 p3 16 p4 17 p5 18 p6 completely filled s & p - very stable Representative Elements The representative elements are the s- and p- block elements d-block elements Same as transition metals In period n • ns2 (filled s sublevel) • Partially filled or filled d orbitals of level (n-1) • d sublevel can hold 10 electrons; dblock spans 10 groups on periodic table d-block elements Period 4 - Filling the n-1 d sublevel • Sc [Ar]3d14s2 • Ti [Ar]3d24s2 Filling is more or less regular but exceptions occur • Cr and Cu irregular because of stability of filled and half-filled d sublevel Period 4, d Block Exceptions Aufbau diagram works to vanadium, AN 23 Half-filled and fully-filled set of d orbitals have extra energy stability, so chromium is Cr [Ar]3d54s1 (1/2 filled d) Not [Ar]3d44s2 Next exception is copper: Cu [Ar]3d104s1 (filled d) Not [Ar]3d94s2 f-block elements f sublevel can hold 14 electrons; f-block spans 14 columns of periodic table Electrons don’t fill orbitals in a predictable manner f-block Inner Transition Metals For period n (n = 6 or 7) • Filled ns (ns2); Filled or partially filled (n-2) f (4f & 5 f) • First member of lanthanides (La, AN 57) & actinides (Ac, AN 89) not in Aufbau order – have d1 configuration, expect f1 • f sublevel filled one element prior to last member of either row Aufbau Order & Periodic Table Can “read” Aufbau order (page 160) directly from periodic table and knowledge of how blocks fill Move left to right Keep period number for representative elements (groups 1-2, 13-18) (Period number -1) for d block (Period number -2) for f block (start after La or Ac to get closer to actual order) s, p, d, and f blocks s, p, d, and f blocks p block s block d block f block Practice Practice problems, page 162 Problems 7-9 Section assessment, page 162 Problems 10-15 Chapter 6 Periodic Table & Periodic Law 6.1 Development of the Modern Periodic Table 6.2 Classification of the Elements 6.3 Periodic Trends Section 6.3 Periodic Trends Trends among elements in the periodic table include their size and their ability to lose or attract electrons • Compare period and group trends of several properties. • Explain the meaning of electronegativity • Relate period and group trends in atomic radii, ionic radii and electronegativity to electron configuration. • Describe the roles of electron-electron repulsion, electronnucleus attraction, shielding (effective nuclear charge), and the added stability of favored electron configurations (octet, half filled and filled sublevels) in determining periodic property trends. Section 6.3 Periodic Trends Trends among elements in the periodic table include their size and their ability to lose or attract electrons • Use Coulomb’s law to explain shielding and its impact on atomic radii. • Predict and explain the change in size that occurs when an atom forms either a cation or an anion. • Predict and explain the relative changes in ionization energy for the first, second, third, etc. ionizations of a given atom. • Explain how departures from overall first ionization energy trends for some elements can occur in terms of the specific electron configurations of these elements. Section 6.3 Periodic Trends Key Concepts • Atomic and ionic radii decrease from left to right across a period, and increase as you move down a group. • Core electrons are effective at shielding valence electrons whereas other valence electrons are not effective shielders. • Ionization energies generally increase from left to right across a period, and decrease as you move down a group. • The octet rule states that atoms gain, lose, or share electrons to acquire a full set of eight valence electrons, which is a particularly stable electron configuration. Filled and halffilled sublevels (particularly d) also impart extra stability. • Electronegativity generally increases from left to right across a period, and decreases as you move down a group. Section 6.3 Periodic Trends Key Concepts • Electronegativity is a property related to bonding and therefore is a measure of an interaction of the atom with another atom. • Disruptions of filled energy levels and of filled sublevels require more energy than removing electrons from other electron configurations. Periodic Trends - Principles Negative electrons are attracted to the positive nucleus Coulomb’s Law: F (+q) (-q) / r2 F = attractive force +q = charge on nucleus -q = electron charge r = distance between charge centers Force vs distance for 1/r2 0 0 Force 0 0 0 0 0 0 0 5 15 25 35 r (distance) 45 Periodic Trends - Principles Coulomb’s Law: F (+q) (-q) / r2 The greater the nuclear charge (+q), the more strongly an electron is attracted The closer an electron is to nucleus (smaller r), the more strongly it is attracted Periodic Trends - Principles Coulomb’s Law: F (-q)(-q)/r2 Force repulsive if charge same sign Electrons repelled by other electrons in an atom The further away two electrons are from each other, the weaker the repulsive force between them Electrostatic Forces in Atom Periodic Trends - Shielding If other electrons are between a valence electron and nucleus, valence electron will be less attracted to nucleus Valence electron r Inner (core) electron +3 F1 (+3)(-1) / r2 +3 F2 (< +3)(-1) / r2 Both forces are attractive, but F2 is smaller than F1 due to shielding Periodic Trends - Shielding If other electrons are between a valence electron and nucleus, valence electron will be less attracted to nucleus – nuclear charge is shielded Effective nuclear charge less than full nuclear charge due to shielding of charge by negative core electrons Other valence electrons not effective at shielding a valence electron Shielding Effect – Mg: [Ne]3s2 RED – attractive forces Core Electron Cloud Representative Core Electron BLUE – repulsive forces Nucleus Valance Electron Periodic Trends - Principles Filled principal energy levels are very stable (noble gas configuration) Atoms prefer to add/subtract/share valence electrons to completely fill a principal energy level if possible (octet rule) Completely filled sublevels (s, p, d) also have extra stability Atomic Radius Sizes mostly obtained from crystalline form of element or from diatomic molecule In general, are averages of internuclear separations observed in a variety of substances Typically expressed in pm = picometer = 10-12 m May also see in nm = nanometer = 10-9 m Atomic Radius Sodium in crystal Bonded sodium atoms 372 pm 186 pm Radius Atomic Radius Hydrogen in gaseous diatomic molecule Bonded hydrogen atoms 74 pm 37 pm Radius Atomic Radius Trends: Figure 6.11 Atomic Radii Trends: Figure 6.12 Group trend – more shielding, more distant orbitals with increasing n Period trend – increasing effective nuclear charge Trends – Atomic Radius Period trend (left to right) – dominated by increasing effective nuclear charge with no change in n and with little additional shielding provided by valence electrons Group trend (top to bottom) – dominated by increasing n (orbitals more distant) and shielding of nuclear charge by core electrons (from filled energy levels) – effective nuclear charge decreases 1s, 2s, 3s Orbitals – Distance From Nucleus Nodes Node 1s 2s 3s 1s 2s 3s Trends – Ionic Radius Ion is charged atom - electrons have been added or removed • Positive ion (+ ion) formed if electrons removed – called cation • Negative ion ( ion) formed if electrons added – called anion Trends – Ionic Radius: Cations When atoms lose electrons and form positively charged ions (cations), they always become smaller for 2 reasons: 1. The loss of a valence electron can leave an empty outer orbital resulting in a small radius 2. Electrostatic repulsion decreases allowing the electrons to be pulled closer to nucleus Trends –Ionic Radius Ionization of elemental sodium Na Na+ + eSodium atom Sodium ion [Ne]3s1 [Ne] Trends – Ionic Radius: Anions When atoms gain electrons and form negatively charged ions (anions), they always become larger because electrostatic repulsion increases, causing the electrons to spread apart If added electron placed in previously unoccupied energy level, then average distance from nucleus will be greater for that electron Trends –Ionic Radius Ionization of atomic chlorine Cl + e ClChlorine atom Chlorine ion [Ne]3s23p5 [Ne]3s23p6 or [Ar] Ionic Radii in Crystal Ionic Radius – Magnesium oxide Trends – Ionic Radius Formation of + ions always results in decrease in radius Formation of - ions always results in increase in radius Electron configuration of ions follows same filling order as for neutral atoms Within a set of + ion or – ions, radius trends for groups/periods are the same as for neutral atoms Ionic Radius Trends See figure 6.14 Ionic Radius Trends – Fig. 6.15 Within a category (+ or – ions), trend is same as trend for atomic radius Comparison of Atomic & Ionic Radii Ionization Energy (IE) Energy needed to remove electron from neutral gaseous atom to form + ion X(g) X+(g) + e- kJ/mol • Ionization potential is per atom value in eV (1 eV=1.602 x 10-19 J) Must overcome electrostatic attraction to remove electron Low IE = easy valence electron loss – element will readily form a cation 2nd Ionization Energy Energy needed to remove electron from ion with single positive charge X+ (g) X2+(g) + e- kJ/mol Must overcome much stronger electrostatic attraction to remove second electron compared to the first electron Trends – Ionization Energy Group 1 lowest values and Group 18 highest values within a period Group 1 likely to form M+1 but unlikely to form M+2 • Difficult to remove electron from noble gas configuration (filled p sublevel) Trends – Ionization Energy (IE) Period and group trends inverse of radii trends – increased nuclear charge pulls electron in tighter Shape of trend for any given period is irregular due to stability of filled / half filled sublevels Trends – st 1 Ionization Energy Periods 1-5 Fig. 6.16 Trends – 1st Ionization Energy Fig. 6.17 Trend opposite that for atomic radius Reasons for trend same as for atomic radius Trends – Ionization Energy (IE) 2nd IE always > 1st 3rd IE always > 2nd etc. • Removing electron from more + ion Once valence electrons removed, energy always takes big jump • Must remove electron from filled level Period 2 Successive Ionization Energies (Table 6.5) Trend Exceptions – (IE) 1st IE (values in kJ/mol) Be (1s22s2) 900 vs B (1s22s22p1) 800 • B has inner 2s2 configuration which effectively shields 2p1 electron N (1s22s22p3) 1400 vs O (1s22s22p4) 1310 • Half-filled p sublevel has extra stability Trend Exceptions – IE 2nd IE B (1s22s22p1) 2430 vs C (1s22s22p2) 2350 • 2s2 effectively shields O (1s22s22p4) 3390 vs F (1s22s22p5) 3370 • Disrupts/creates 2p3 configuration Trends - Electronegativity Relative ability to attract electrons in a chemical bond Max 3.98 (F) to min 0.7 (Fr) Elements with high EN tend to form negative ions • F-, Cl-, O2- Noble gases not tabulated • Very few compounds to get info from Decreasing Electronegativity Trends - Electronegativity Increasing Electronegativity electronegativity < 1.0 1.0 electronegativity < 2.0 2.0 electronegativity < 3.0 3.0 electronegativity < 3.9 electronegativity 3.9 Trends – Electronegativity vs AN Trend Summary (ignore EA)