* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Adverse Effects of Antiepileptic Drugs
Survey
Document related concepts
Adherence (medicine) wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Atypical antipsychotic wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Pharmacognosy wikipedia , lookup
Plateau principle wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Transcript
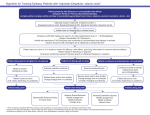
Epilepsia, 41(Suppl. 2):S42-S52, 2000 Lippiiicott Williams & Wilkins, Inc., Baltimore 0 International League Against Epilepsy Adverse Effects of Antiepileptic Drugs Robert S. Greenwood Department of Neurology, University of North Carolina School of Medicine, Chapel Hill,North Carolina, U.S.A. Summary: Because the efficacies of antiepileptic drugs (AEDs) are often equivalent, selection of an AED is often determined by adverse effects. Differences in methods for labeling adverse effects and in the adverse effect terms themselves, variations in the populations studied, and inconsistent classifications of adverse effects make it difficult to know how to use information on adverse effects to choose an AED. Effort is underway to develop more extensive and internationally acceptable descriptive terms for adverse effects. Comparison of adverse effects in patients taking AEDs with adverse events in control groups is helpful; however, data from controlled studies are often lacking for most AEDs. Because of these limitations, the clinician must adopt a preventative and early detection approach based on some general principles. This review outlines factors to consider for avoiding and detecting AED adverse effects. The occurrence of weight change with AEDs is reviewed extensively, serving to illustrate how the principle factors can be used to avoid and manage adverse effects and where there is need for better studies of the short- and long-term adverse effects of AEDs. Key Words: Antiepileptic drugsEfficacy-Adverse effects-Weight changes-GABA. Recent attempts to develop better ways of choosing antiepileptic drugs (AEDs) have placed emphasis on the inclusion of adverse effects in the analysis. Doctors who treat patients with epilepsy are now attempting to develop methods of choosing AEDs that factor in existing information about efficacy, adverse effects, cost, and issues of administration (1,2). The efficacies of AEDs are often found to be equal in comparative studies; therefore, AED selection is largely determined by adverse effects (3). Definitions and measurements of adverse effects have been inconsistent and, consequently, the methods by which adverse effects are defined and detected are changing. The growing list of new AEDs has also increased the need to compare AED risks. Although the dose-related adverse effects of new AEDs seem to be less, the new AEDs have introduced new adverse effects that require added vigilance during the chronic use. Adverse effects have also become major factors in the overall cost of epilepsy treatment (4). For all of these reasons, it has been difficult for the physician caring for patients with epilepsy to use adverse effects to help in the choice of an AED. DEFINITION OF ADVERSE EFFECTS The definition and descriptive terms for adverse effects vary among AEDs. Several terms are used to describe health problems that arise in patients taking AEDs, including “adverse events,” “adverse experiences,” “problem rates,” “complaint rates,” “adverse effects,” and “side effects.” The terms “adverse effects” and “side effects” are often used in publications summarizing AED adverse events. “Adverse events” and ‘‘adverse experiences” are used interchangeably to refer to the occurrence of an undesirable patient event during use of a drug. These terms intentionally avoid implying a necessary cause-and-effect relationship between the use of the drug and the event. The Food and Drug Administration (FDA) has defined a reportable adverse drug experience as “any adverse event associated with the use of a drug in humans, whether or not considered drug related” (5). They include adverse events that occur during the use of the drug in professional practice or drug study, during a drug overdose, as a result of drug withdrawal, and as a result of the failure of “expected pharmacological action.” The FDA attempts to further classify these adverse events as possibly, probably, or definitely related to the use of the AED by using data from many different sources and attempting to judge the importance of that data. The terminology used in the United States to describe patient reports of adverse experiences in most clinical trials of new AEDs comes from Coding Symbols for a Address correspondence and reprint requests to Dr. Robert S. Greenwood at the Department of Neurology, University of North Carolina School of Medicine, CB# 7025 Burnett-Womack, Chapel Hill, NC 27599, U.S.A. S42 ADVERSE EFFECTS OF ANTIEPILEPTIC DRUGS Thesaurus of Adverse Reaction Terms (COSTART), a thesaurus of terms for describing patient complaints. Studies vary widely in the use of COSTART terms and how the complaints are obtained. The use of COSTART is not universal, and a list of AED adverse effects may include terms taken from other sources, such as the World Health Organization’s Adverse Reaction Terminology (WHO-ART), International Classification of Diseases (ICD) 9, and ICD9-CM. To improve the terminology used to describe adverse effects, the FDA plans to replace COSTART with the Medical Dictionary for Regulatory Activities (MedDRA) (6). The International Conference on Harmonization, an organization made up of representatives of industry associations and regulatory authorities in United States, Europe, and Japan, has agreed upon the structure and content of the MedDRA so that this terminology may be used internationally. The descriptive terms used for adverse effects will be expanded to nearly 10 times the 1,200 terms used in COSTART, and MedDRA will have a hierarchical structure to allow greater specificity (6). Improvements in the number of descriptive terms for adverse effects and international use of these terms will make it easier to compare adverse effects when selecting AEDs. Variations in labeling adverse effects and the use of different descriptive terms, as noted previously, create problems in comparing adverse effects of AEDs. Another problem with current reports of adverse effects is that objective quantifiable measures of most complaints are not used frequently in studies of drugs. This also makes comparisons of AED adverse effects among studies difficult. To allow better comparison of adverse effects, the terms “problem rates” and “complaint rates” have been proposed by some doctors who treat patients with epilepsy to summarize information on new AEDs (2). The “problem rate” is the percentage of patients taking an active drug or placebo that report an adverse experience. The “complaint rate” is the problem rate for patients receiving active treatment minus the problem rate for patients receiving placebo. Use of the complaint rate helps control for variations in patient populations and data collection in different studies. The FDA also has suggested the use of other statistical methods to allow comparison of adverse effects from different studies. Recommended statistical methods include relative risk (cumulative risk on drugkumulative risk on comparison drug or placebo) or attributable risk (cumulative risk on drug - cumulative risk on comparison drug or placebo). Unfortunately, as Cramer et al. (2) have noted, the lack of uniformity of populations, terminology, methods for collecting, and patient experiences and the absence of formal methods for testing for adverse effects make comparisons of complaint rates, relative risks, and attributable risks among studies difficult. In addition, the designs of many studies, especially long-term studies, do s43 not include a placebo group or a comparison drug. An alternative method that may be used to compare adverse effects when no comparison group is available is the dropout rate. When the data are available, rate of dropout due to adverse effects may allow better judgement of the importance of dose-related or idiosyncratic adverse effects. Unfortunately, often these data are difficult to compare in many AED studies because doses may be lower than those used in clinical practice and the length of study may be too short. Similar to the comparison of AED efficacy, all of these problems make the comparison of AED adverse effects imperfect. When considering adverse effects to choose an AED, the clinician must consider each of the problems confounding the comparison of AED adverse effects. When available, the difference between rates of adverse effects in the AED group and placebo group and rates of dropout may be the most reliable and comparable adverse effect rates. The clinician should also remember that the true incidence of adverse effects can be obtained only after many years of AED use in a varying clinical setting. CLASSIFICATION OF ADVERSE EFFECTS The classification systems for AED adverse effects provide the clinician with ways to remember different adverse effects. AED adverse effect classification systems may also help the clinician prevent or treat adverse effects. Table 1 lists several ways in which adverse effects have been classified. The classification “doserelated” or “idiosyncratic” is most commonly used, because it identifies the drug as the cause of the adverse effect and, in the case of “dose-related,’’ implies that the adverse effect will improve with dose reduction. Classification of adverse effects by organ system may help identify organs that must be monitored clinically and experimentally. Perhaps the most advantageous classification of adverse effects is based on the mechanism involved in the adverse effect. This classification scheme suggests what organ systems to monitor for adverse effects and how to detect them as well as how adverse effects may be treated. Unfortunately, the mechanisms for most of adverse effects are so poorly understood that most adverse effects can not be classified in this manner. TABLE 1. Classifications of adverse events FDA Dose-related Organ systems Mechanism Life-threatening, serious, unexpected Dose-related, idiosyncratic Cardiovascular, gastrointestinal and hepatic, hematologic and lymphatic, endocrine/metabolic, musculoskeletal, nervous system, special senses, respiratory, dermatoiogic, genitourinary, reproductive Metabolickoxic, endocrine, immune, teratogenic, oncogenic, apoptotic s44 R. S. GREENWOOD The confusing descriptive terms and varying classification schemes for adverse effects make it difficult to know how to use this information to select an AED and monitor a patient. Table 2 lists AED adverse effects that are especially common or unique. This list is a starting point for considering AED adverse effects. An efficient way to extend the consideration of adverse effects in the choice of an AED is by being aware of the windows of susceptibility. Following is a discussion of some of the most important factors to keep in mind when considering the potential adverse effects of an AED. IMPORTANT FACTORS TO CONSIDER IN EVALUATING ADVERSE EFFECTS Some AEDs worsen seizures Many adverse effects of AEDs relate directly to their therapeutic effects, as noted above. This includes doserelated adverse effects, and even some idiosyncratic adverse effects. AEDS may even increase seizure frequency or severity or change the seizure type (Table 3). It may be difficult to be certain that an AED exacerbates or worsens a seizure type, particularly with partial seizures, but accurate counts of seizures preceding the inTABLE 2. Especially common or unique adverse effects of AEDs Especially common or unique adverse effects Drug Phenytoin Barbiturates Suximides Carbamazepine and oxcarbazine Carbamazepine Benzodiazepines Valproate Felbamate Gabapentin Lamotrigine Topiramate Tiagabine Vigabatrin Zonisamide Cosmetic changes (gum overgrowth, coarsening of facies, hirsuitism), peripheral neuropathy, hypersensitivity reactions Behavioral changes and cognitive effects, Dupytren’s contractures, peripheral neuropathy Hiccups, gastrointestinal problems, visual changes Hyponatremia Leukopenia, drug eruptions Sedation, hypersalivation Weight gain, endocrine changes, tremor, hair loss, thrombocytopenia, hyperammonemia, hepatotoxicity, pancreatitis Anorexia and weight loss, insomnia, aplastic anemia, liver failure Hyperactivity or aggressive behavior in children, weight gain Skin rash Cognitive problems (speech disturbance), behavioral abnormalities, kidney stones, paresthesias, anorexia, weight loss Tremor and nervousness, emotional changes Behavioral and psychiatric disturbances: irritability, insomnia, hyperactivity, psychosis and depression, visual field defects and blindness Kidney stones Epilepsia, Vol. 41, Suppl. 2, 2000 TABLE 3. Antiepileptic drugs reported to increuse seizures Antiepileptic drugs Seizure type that increases Carbamazepine, phenobarbital, vigabatrin, gabapentin Carbamazepine, vigabatrin, gabapentin, lamotrigine Carbamazepine, phenytoin, vigabatrin Carbamazepine Absence Myoclonic Complex partial Tonic-atonic Information found in references 7 and 1 1. troduction of a new AED may help the clinician identify this adverse effect. AEDs that increase GABA levels may worsen or exacerbate some generalized seizures (7). Vigabatrin worsens myoclonic and absence seizures (7,8), and worsening of myoclonic seizures also has been noted with gabapentin (9) and with lamotrigine in the treatment of severe myoclonic epilepsy of infancy (10). Carbamazepine can increase absence seizures (7). Focal seizures often increase when vigabatrin controls partial seizure generalization (1 1). In many patients beginning treatment with AEDs for control of partial seizures with secondary generalization or complex partial seizures, seizure manifestations change as the seizures come under control. Partial seizures with secondary generalization may become complex partial seizures or simple partial seizures, and complex partial seizures may evolve to simple partial seizures. In one study, valproate treatment of an adult with a hypothalamic tumor and seizures was reported to result in confusion and increased spike and wave activity that resolved when the medication was discontinued (1 2). Pathological laughter, a common feature of seizures associated with hypothalamic tumors, may occur with intravenous administration of valproate (13); therefore, use of valproate in the treatment of these types of seizures must be done with added care. Toxic levels of some AEDs, such as phenytoin, can increase seizures that were controlled initially (14). Psychosis as a treatment-emergent effect with the start of vigabatrin treatment has been correlated with a right-sided EEG focus and seizure control and often with complete control of overt seizures (15,16). AED withdrawal, especially when abrupt, and especially with felbamate (17) and vigabatrin (1 5), can also result in transient increase in seizures or even status epilepticus. Because AEDs may worsen seizures, one of the first considerations in choosing an AED is the efficacy for the patient’s type of seizure or epilepsy. Age and sex Age and sex are important factors in the occurrence of adverse effects. Some of the endocrine and reproductive adverse effects are obviously different for men and women. There are some conspicuous examples of age as an important factor in the occurrence of adverse effects with other drugs. Increased drug toxicity in young pa- ADVERSE EFFECTS OF ANTIEPILEPTIC DRUGS tients occurs with the use of chloramphenicol in the newborn or tetracycline in infants and children. In contrast, adults have greater toxicity from aminoglycosides. The adverse effects of AEDs also vary with age. The growth effects of AEDs are important in children but not in adults. Elderly patients are not only more responsive to AED therapy, but also are more likely to have doserelated adverse effects, often at lower drug concentrations (18). This reflects, in part, the pharmacokinetic changes and lower serum albumin in elderly adults. Liver volume, blood flow, and metabolism decrease with age (19). Elderly adults or neurologically impaired infants and children also may be more susceptible to some doserelated adverse effects, such as dizziness, because of preexisting neurological problems. Some types of seizures (but not usually epileptic syndromes) in one age group may be made worse by the same AED that controls them in another age group. For example, vigabatrin reduces myoclonic seizures of infancy (infantile spasms), and yet worsens myoclonic seizures in older patients with other types of epilepsy (7). Serious hematological complications are rare with AEDs but seem to be more common in older patients (20). In a cohort study of serious blood dyscrasias in patients 10 to74 years of age who were taking AEDs, the overall rate of blood dyscrasias was 3 to 4 per 100,000 prescriptions. The rate in patients <60 years of age was 2.0 (range 0.9 to 3.6) per 100,000 prescriptions, compared with 4.0 (range 1.6 to 8.2) per 100,000 prescriptions for patients >60 years of age (20). The high rate of aplastic anemia in older patients treated with felbamate seems to be less in children (21). Infants and young children, however, are at greater risk for other adverse effects. The occurrence of StevensJohnson syndrome was higher in patients taking AEDs who were <18 years of age (22). Children <2 years of age are at greater risk for hepatotoxicity associated with valproate (23). Instances where infants and children have a greater susceptibility to an AED adverse effect often are related to inherent susceptibility, which is another factor that should be considered when evaluating AEDs. Inherent susceptibility Inherent susceptibility refers to genetic differences in some patients with epilepsy that predispose them to an AED adverse effect. Some of the most obvious of these are found in the contraindications listed in the drug insert for each AED. One of the most prominent examples of inherent susceptibility is porphyria. The AEDs that induce hepatic metabolism may precipitate a worsening of symptoms in patients with porphyria. In some instances, this may even include exacerbation of seizures. Other inherent susceptibilities, such as the disorder that increases the rate of valproate hepatotoxicity with the use of valproate in children <2 years of age, are not as ex- s45 plicit. This increased susceptibility to valproate hepatotoxicity in children may in part reflect underlying energy metabolic defects (24,25). Valproate interferes with mitochondrial metabolism through mechanisms that sequester coenzyme A or inhibit mitochondrial P-oxidation enzymes. Severe impairment of mitochondrial p-oxidation can lead to microvesicular steatosis, liver failure, coma, or death (26). Even in adults with fatal hepatotoxicity, proven or suspected mitochondrial disorders have been more common (27). Variation in the regulation and expression of the drug-metabolizing enzymes may also play an essential role in both interindividual variation in sensitivity to drug toxicity and tissue-specific damage (28). Genetic or environmental impairment of cytochrome P450 activity may lead to toxicity. Active metabolites produced by cytochrome P450 enzymes may cause a variety of pathologic effects, including cellular necrosis, hypersensitivity, teratogenicity, and carcinogenicity (28). The leading hypothesis for the pathogenesis of AED hypersensitivity reactions is that the AED is oxidatively metabolized to reactive-cytotoxic intermediates that interact covalently with cellular macromolecules to form haptens (29). In this scheme, the haptens are immunogenic but the original AED is not. Unfortunately, no identifiable differences in detoxification pathways or immune effectors that predict susceptibility to hypersensitivity reactions have been identified (30). Efforts to determine the inherent susceptibilities for many of the serious adverse effects of AEDs are underway. Continued advances in genetics and molecular biology may lead to ways of identifying patients susceptible to AED adverse effects so that we can avoid exposing them AEDs or take measures to counteract susceptibility to adverse effects. For now, the clinician must depend on clues from the patient’s history and family history to suspect an inherent susceptibility. Importance of preexisting conditions Many diseases increase the risk of AED adverse effects. In some cases, preexisting conditions are simply a version of the propensity wrought by an inherent susceptibility. In other cases, the increased susceptibility comes from acquired diseases. Dose-related and idiosyncratic adverse effects may occur at greater rates with preexisting conditions. Patients with hepatic disease that reduces protein synthesis are more likely to develop adverse effects with drugs that have high protein binding, such as phenytoin and valproate. In addition, AEDs that are primarily detoxified in the liver, such as barbiturates, phenytoin, suximides, carbamazepine, felbamate, lamotrigine, tiagabine, valproate, and benzodiazepines, may rise to toxic levels in patients with liver disease. For patients with liver disease, these drugs offer greater risk and may not be the first choice, or doses may require close monitoring. To avoid dose-related adverse effects Epilepsra, Vol. 41, Suppl. 2, 2000 S46 R. S. GREENWOOD in patients with renal disease, doses of AEDs with predominantly renal excretion, such as gabapentin and topiramate, must be reduced or the AED should be avoided. Some idiosyncratic AED adverse effects have occurred at high rates in patients with preexisting systemic illnesses. Patients with aplastic anemia during felbamate treatment frequently have prior histories of immunemediated disorders, drug rashes, and autoimmune disease (31,32). Patients who have had prior drug rashes are more likely to develop AED rashes. This phenomenon of cross-reactivity is most obvious in the increased risk of worsening or recurring AED rash after a drug rash from a prior AED. Drug allergy cross-reactivity among many of the aromatic AEDs, such as phenytoin, phenobarbital, primidone, and carbamazepine, may be as high as 70% to 80% (33). Among the new AEDs, lamotrigine has one of the highest incidences of immune-mediated drug eruptions. Lamotrigine has an aromatic ring structure. The drug eruptions with lamotrigine are similar to those that occur with other AEDs, raising the possibility that there could be some cross-reactivity between lamotrigine and aromatic AEDs (34). Valproate has not been reported to have cross-reactivity with other AEDs and is rarely associated with immune-mediated adverse effects (34) and thus may be the AED of choice during some immunemediated adverse effects. Some studies have suggested that patients with brain tumors who receive radiation therapy may be at greater risk for allergic skin reactions to AEDs (35,36). In one case, the eruption during phenobarbital treatment was limited to the sites of radiation (36). Another study of skin eruptions in patients with brain tumors receiving cranial irradiation suggested that factors related to the brain tumor may increase the risk of skin eruptions with the use of AEDs. In this study, mild rashes occurred in 18% of exposures to AEDs, including 22% of exposures to phenytoin, compared with the expected rate of 5% to 10% (37). Most of these rashes occurred before initiating cranial irradiation. AED rashes were especially likely when steroids were being tapered. These examples represent only a few of the conditions that increase the frequency or severity of AED adverse effects. In general, the use of AEDs requires the greatest caution in the patients with the most preexisting conditions. Greatest risk for adverse effects is at start of treatment Most AED adverse effects occur at the start of AED treatment, often because of the predisposing factors previously noted. Dose-related adverse effects are more prominent at the start of treatment and often improve or even resolve over time without a change in AED dose. The dose-related adverse experience rates available from clinical trials are some of the most difficult to interpret. Not only do the reports vary for reasons cited previously, but studies of AEDs in early clinical trials were designed with incomplete knowledge of potential AED adverse effects or effective AED doses. In controlled trials of AEDs that study different doses, many adverse effects have been shown to be dose related. These types of studies may help provide guidance in choosing doses to avoid adverse effects. Weighing the importance of a dose-related adverse effect, however, is one of the arts of medicine. Tolerance and adaptation to dose-related AED adverse effects will vary widely among patients for many reasons, which include not only medical factors but also the patient’s culture, marital status, need for specific high levels of function, expectations, and financial concerns, Some of the most important idiosyncratic reactions also occur at the start of AED treatment. Idiosyncratic reactions after the start of treatment, such as hypersensitivity reactions and hepatic adverse effects, are most likely to occur within 2 to 8 weeks (33). Because many adverse effects occur most frequently at the start of treatment, clinicians should be most vigilant during this time. Patients and their families should be instructed to be particularly vigilant about looking for adverse effects during the first weeks of a new AED treatment and should be encouraged to contact the physician quickly if they have concerns about an adverse effect so that the severity of these complications may be reduced. Rapid dose changes, high AED doses, polypharmacy, and other treatments increase risk of adverse effects Many AED adverse effects, especially dose-related, are more frequent and severe when AEDs are initiated rapidly or administered at high doses. Loading with AEDs, as occurs during the treatment of status, will invariably increase dose-related adverse effects. Doserelated adverse effects of AEDs may occur in individual patients even when AED levels are within a therapeutic range. This problem arises, in part, because therapeutic blood levels are statistical best estimates of the AED levels that produce toxic effects and are often derived from small samples of patients. Even adverse effects that do not seem directly related to the antiepileptic effect of an AED, such as thrombocytopenia with valproate treatment, are dose related (38). Dose-related adverse effects also often increase when multiple AEDs are combined. The use of other drugs or coadministration of two or more AEDs may also increase the toxicity of an AED. Pharmacokinetic interactions and changes in pharmacodynamics are common when antiepileptic drugs are used together (39) or when coadministered with other medications. Drug interactions are listed in the drug inserts to warn the physician about the risks of drug interactions. Many AEDs are metabolized in the liver and cause induction or inhibition of liver enzymes. Carbamazepine, ADVERSE EFFECTS OF ANTIEPILEPTIC DRUGS phenobarbital, phenytoin, and primidone induce some liver enzymes, whereas valproic acid inhibits them (40). Other drugs (e.g., aspirin, tetracyclines, several 2-arylpropionate anti-inflammatory drugs, amineptine, and tianeptine) that interfere with mitochondria1 metabolism in a manner similar to valproate may enhance valproate hepatotoxicity (26). Idiosyncratic adverse effects often increase with polypharmacy or rapid dose escalation. Polypharmacy is associated with an increased risk for valproate hepatotoxicity (41) and aplastic anemia during felbamate treatment (3 1,32). The use of valproate with lamotrigine and rapid lamotrigine dose escalation greatly increases the risk of skin eruptions (22). In general, adverse effects can be avoided or severity can be reduced by slowly increasing the AED dose and avoiding polypharmacy. This review of AED adverse effects is only a broad overview of a very complex topic. The issues and factors reviewed can be used in conjunction with the lists of AED adverse effects available from a variety of sources, such as drug inserts and Physicians’ Desk Reference. Detailed reviews of AED adverse effects can also be found in recent volumes of standard epilepsy books (4247). What follows is a description of one adverse effect: weight changes that accompany the start of treatment with an AED. NEW ATTENTION TO WEIGHT CHANGES AS AED ADVERSE EFFECT Doctors who treat patients with epilepsy recognize that limiting AED adverse effects is one of the most important goals in optimal treatment of epilepsy. Recently, studies of weight changes in patients taking AEDs and studies attempting to define the mechanisms that account for these changes have appeared. These studies are an example of approaches to understanding, preventing, and managing adverse effects that consider many of the factors discussed in the preceding section. These studies also emphasize the importance of age, sex, drug dose, preexisting conditions, and inherent susceptibility to body weight changes associated with AEDs. Studies of weight changes show how the antiepileptic properties of AEDs may play a direct role in an adverse effect usually considered idiosyncratic. A review of the mechanisms for regulation of body weight will follow, providing a framework for understanding how AEDs and seizures might affect body weight. Regulation of body weight Much is known about the control of body weight. Some mechanisms for weight control implicate pathways, neurotransmitters, and receptors that could be influenced by AEDs. The control of body weight and mass involves multiple mechanisms. Body weight changes depend on the balance of caloric intake and energy ex- s47 pended. The brain integrates information from many afferent inputs and circulating factors to regulate food intake and energy expenditure. The hypothalamus is the principle site of this integration (Figure 1). Signals arising from food ingestion and metabolism and social and emotional influences, acting through the brain stem and limbic system, impinge on the hypothalamus where they influence eating and energy expenditure. Energy metabolic rate and activity level influence caloric expenditure. Figure 1 illustrates the hypothalamic nuclei involved in regulating food intake, energy expenditure, and intrinsic and afferent pathways and messengers that influence these nuclei. Afferent pathways and circulating neuromodulators influencing caloric intake include olfactory and oral special and somatic sensory input, stomach and intestinal stretch receptors input, CCK and other neuropeptides released from the intestinal tract acting through the vagal nerve afferents, glucose levels affecting glucose-sensitive neurons in the brain and gut, and leptin and glycerol released from adipose tissue to act on the lateral hypothalamus (48). Serum leptin and glycerol concentrations are correlated with the percentage of body fat (49). Two areas of the hypothalamus integrate the afferent signals to regulate feeding patterns: the lateral hypothalamus and the medial hypothalamic nuclei. Caloric balance may be altered, with ventromedial lesions causing hyperphagia and lateral hypothalamic lesions producing a syndrome of aphagia and weight loss in experimental animals (50). From these types of studies, the medial hypothalamic region has been designated the “satiety center” and the lateral hypothalamic region the “feeding center.” Neurotransmitters, neuromodulators, and hormones influence the activity of the neurons in the hypothalamus. y-aminobutyric acid (GABA) and neuropeptide Y increase carbohydrate consumption and reduce energy expenditure (51). Serotonin acts on the medial hypothalamus to reduce food ingestion. Dietary increases in carbohydrates increase 5-HT levels. Norepinephrine, when injected in the paraventricular nucleus, elicits eating behavior, whereas injection of dopamine or P-adrenergic drugs in the lateral hypothalamus reduces feeding. Caloric expenditure is influenced by basal metabolic rate, thermogenesis, and physical activity. Basal metabolism is influenced by inherited traits, some of which may be specific to the caloric substrate (fat, sugar, or protein) or involve hereditary alterations in leptin or glucoseresponsive neurons. Activity level is affected by social and emotional factors, level of consciousness, and fatigue. Changes in metabolic rate are also influenced by circulating hormones and growth factors, especially thyroid hormone and insulin-like growth factors. The satiety and feeding centers of the hypothalamus and other areas of the brain important in regulation of body weight can be affected indirectly or directly by anticonvulsants. S48 R. S. GREENWOOD FIG. 1. This diagram illustrates the hypothalamic nuclei that integrate input from many afferent pathways (solid arrows) and from circulating substance (dashed arrows). The hypothalamus receives and sends messages from many brain stem and spinal cord nuclei as well as from the limbic system and cerebral cortex. The nucleus tractus solitarius in the brain stem plays a pivotal role in transferring information from the autonomic system and sensory stimuli received through cranial nerves (CN) to the hypothalamus and limbic system. In a simplified view of the hypothalamus, the efferent activity of the ventromedial nucleus (VMN) and paraventricular nucleus (PVN) inhibit feeding, whereas the efferent activity coming from the lateral hypothalamic nuclei (LHN) predominantly activates feeding behavior. The neurotransmitters y-aminobutyric acid (GABA) and norepinephrine (NE) increase feeding (t).In the case of NE, intake of carbohydrates (CHO) is primarily increased. The neurotransmitters, serotonin (5-HT) and dopamine (DA), have been shown to primarily reduce feeding (J). Neuropeptides that affect the hypothalamus to influence feeding include vasopressin (VP), proopiomelanocortin(POMC),cholecystokinin (CCK), and gastrin-releasing peptide (GRP) that reduce feeding. Feeding is increased by the neuropeptides, neuropeptide Y (NPY), agouti-related peptide (AGRP), and galanin (GAL), but NPY increases CHO intake whereas GAL increases fat intake. Leptin is thought to act on the LHN to inhibit feeding behavior, but receptors ( + ) for leptin are found in many nuclei of the hypothalamic and limbic system. The hypothalamus also contains glucose-sensitive neurons (GSNs) located predominantly in the LHN and glucose responsive neurons (GRNs) located predominantly in the nuclei of the medial hypothalamus. Glucose reduces the activity of GSNs ( 0 )and increases the activity of GRNs perhaps thereby leading to a reduction in feeding behavior. Amino acid-sensitive neurons in the APC also act to inhibit feeding in response to elevation of circulating amino acids (AA). Other circulating factors that affect feeding behavior include enterostatin, glucagon-like peptide-1 (GLP-l), glucocorticoids, and insulin. APC, anterior piriform cortex; DMNIVMN, dorsomedial nucleus and ventromedial nucleus; LHN, lateral hypothalamic neurons; PVN, paraventricular nucleus; SCN, suprachiasmatic nucleus; 3rd Vent, third ventricle. AED-associated weight changes Weight gain and weight loss in children and adults are disturbing general health and cosmetic adverse effects. Both weight gain and weight loss have been associated with AEDs (Table 4) and may involve some of the same pharmacological mechanisms that play a part in seizure control (Figure 1, Table 5). Weight changes may also play a role in the occurrence of other adverse effects, such as polycystic ovaries in women taking valproate (52). Weight changes are chronic adverse effects that usually appear slowly, and they can easily be overlooked by physicians unless careful measurements are done at regular intervals. Excessive weight gain can be measured by anthropometric parameters; body mass index (BMI) is most commonly used. BMI is calculated from the body weight in kilograms divided by the square of body length in meters. In persons >19 years of age, obesity is often defined as BMI equal to or greater than 27.8 for men and 27.3 for women. In adolescent boys, obesity is defined as BMI equal to or greater than 23.0 for ages 12 to 14 years, 24.3 for ages 15 to 17 years, and 25.8 for ages 18 to 19 years. In adolescent girls, obesity is defined as BMI equal to or greater than 23.4 for ages 12 to 14 years, 24.8 for ages 15 to 17 years, and 25.7 for ages 18 to 19 years (53). The 85th percentile is used as the upper limit for normal weight; obesity is ~ 1 2 0 %of desirable body weight. Actual measurement of body fat content is a more precise way to characterize obesity, but this measurement is too difficult to do on a routine basis. Regular measurements of body weight and length and, in some cases, calculation of BMI provide a quantitative way to monitor an adverse effect that has great impact on quality of life. These measurements should be done as part of the TABLE 4. A. AEDS associated with weight gain Patients with weight gain Drug Gabapentin Adults Children Adults Vigabatrin Adults Valproate Children Carbamazepine 7% to 73% (60) 10% to 44% (60) 8.8% (75) 3% to 46% (76) 7% in clinical trials (4% in placebo group) (75) 5.7% (77) 29% to 43% (60,78) B. AEDs associated with weight loss Patients with weight loss Drug Felbainate Adults Children Topiramate Adults Children 3.4% (79) 4% (81,82) 6.5% (79) 2% (80) 4.4% to 15% (84) 9.2% (83) ADVERSE EFFECTS OF ANTIEPILEPTIC DRUGS TABLE 5. Potential mechanisms of AED-associated weight gain Drug Valproate Neurotransmitter mechanisms GABA Catecholamines Gabapentin Vigabatrin Carbamazepine Tiagabine Metabolic mechanisms 1. Decreased @-oxidation of FFA and gluconeogenesis, secondary to carnitine deficiency 2. Decreased palmitate binding 3. Lower blood glucose GABA GABA GABA NE 5-HT GABA GABA, y-aminobutyric acid; FFA, free fatty acid; NE, norepinephrine. monitoring of patients with epilepsy to detect changes before there are serious adverse consequences. A list of AEDs reported to produce weight gain and weight loss is given in Table 4. The true incidences of these complications are hard to determine in most studies. Some data in Table 4A come from studies with small sample sizes and imprecise measurements and definitions, resulting in a large range of percentage of patients who gain weight. Even in controlled drug trials, study duration is usually not sufficiently long to detect anything other than large changes in weight. Conversely, tolerance to an AED may extend to weight changes, causing weight to change during the early phases of AED treatment and then stabilize or return to pretreatment values. All these factors indicate that values for weight change must be viewed primarily as relative and applicable only to the populations from which they were derived. AED weight gain The drugs most clearly associated with weight changes are valproate and felbamate (Table 4). Valproate causes significant weight gain in many patients, and felbamate causes weight loss. Weight loss associated with felbamate will be discussed below. Weight gain with valproate exceeds that of any other AED associated with weight gain in comparative trials (52,54). In one study, women who gained weight with valproate treatment lost weight when switched to lamotrigine (55). Weight gain has been associated with a significant increase in BMI (52,56) and has been reported in children and adults treated with valproate (52,57). In children, increase in weight and BMI has been significantly positively correlated with initial weight (57). Weight gain has been shown to be significantly greater in prepubertal and pubertal women taking valproate than in age-matched control. In contrast, weight gain in patients taking carbamazepine and oxcarbazepine has not been shown to be significantly greater than that in healthy control girls (58). s49 In a retrospective study of 260 children taking carbamazepine or valproate, however, no significant difference in weight gain was noted, although there were more reports of weight gain as an adverse effect in children treated with valproate (59,60). Weight gain associated with valproate does not seem to abate with continuation but may improve with dosing modification or dose reduction (59,61). No correlation has been found between weight gain and serum concentration, drug dose, appetite, or family history (61). In one prospective study, weight loss and a significant decrease in BMI occurred when patients treated with valproate changed to lamotrigine (55). In a study of patients with juvenile myoclonic epilepsy, weight loss did not occur after changing to lamotrigine (62). Overall, most studies indicate that valproate can be expected to cause weight gain that may result in significant obesity. This seems to be especially likely in older children and adults. Weight gain with valproate treatment may have additional significant associations or effects, some of which may be related to the pathogenesis of weight gain. Isojarvi and colleagues have studied weight change in women and children taking AEDs (52,55). In one retrospective study, they compared weight gain in women taking no AEDs, carbamazepine, and valproate (52). Obesity was found in 59% of women taking valproate, 25% taking carbamazepine, and 21 % of controls. Polycystic ovaries were found in 71% of women with obesity taking valproate but only 13% of the women without obesity taking valproate. Isojarvi et al. also found that these women had high concentrations of fasting serum insulin and low levels of serum insulin-like growth factorbinding protein 1. In a subsequent study ( 5 3 , Isojarvi et al. showed that a decrease in weight, body-mass index, and fasting serum insulin and a reduction of polycystic ovaries followed a change from valproate to lamotrigine. In a study by Rattya et al. (58) of prepubertal and pubertal girls taking valproate, carbamazepine, or oxcarbazepine, there was no change in fasting serum insulin, IGF-binding protein- 1, or IGF-binding protein-3 concentrations. In this same study, plasma insulin-like growth factor-I (IGF-I) levels were higher in girls treated with CBZ and OXC than in girls who served as controls, but these girls did not show excessive growth, which is the expected effect of elevated IGF- 1. Other AEDs are associated with less weight gain (Table 4A) and often only pose a problem at the start of medication. Weight gain is less common in patients treated with gabapentin, and previous weight gain in patients taking AEDs does not predict weight gain with gabapentin. It has been noted that higher dosing increases the likelihood of weight gain. Weight gain with gabapentin may not plateau and continue beyond the first year. Weight gain with vigabatrin treatment has been a consistent finding in most studies. In a study comparing S50 R. S. GREENWOOD vigabatrin with carbamazepine (59), vigabatrin was more likely to be tolerated and had fewer withdrawals, but it was more frequently associated with weight gain (25 lb [11%] vs. 12 lb [5%]). The VA comparative study (54) also showed that weight gain with carbamazepine is less than that with valproate. In this prospective, blinded study, weight gain of <5.5 kg (12 lb) was found in 20% of patients taking valproate versus 8% of patients taking carbamazepine (p < 0.001). Weight gain with carbamazepine treatment has been a consistent finding in most studies, however. Weight gain with carbamazepine is more gradual than with valproate, and increased appetite and excessive food intake may account for increase in weight (63). Carbamazepine may cause mild weight gain, but there is not an untreated group of patients with seizures to use for comparison, and so it is difficult to be confident about this conclusion. Weight gain occurs in patients with seizures who take carbamazepine, gabapentin, and vigabatrin. The weight gain with vigabatrin seems to be a problem, perhaps exceeding that of carbamazepine. It is still uncertain if the weight gain associated with gabapentin is a significant problem. AED weight loss Felbamate and topiramate are the principle AEDs associated with weight loss (Table 4B). Felbamate causes weight loss more frequently than other AEDs. Weight loss may occur in most children taking felbamate, but it may be transient, diminishing over time (64). In one study of 65 patients, who took felbamate for an average of 23 weeks (65), 75% lost weight. Weight loss was greater in adults than in children (4.1% vs. 1.8%, respectively). Weight loss associated with topiramate was greater with higher doses of topiramate and was usually transient (66). In one study of topiramate (67), weight loss was noted early, peaked at 15 to 18 months, and was followed by a slow weight gain. Weight loss was greatest in women and patients who weighed the most at the start of treatment. In summary, weight loss with felbamate and topiramate can be significant enough to lead to discontinuation of these AEDs, but in most instances, weight loss is minimal and transient. Potential AED mechanisms influencing regulation of body weight Table 5 lists possible ways AEDs could affect hypothalamic regulation of body weight. Experimental evidence is beginning to appear that addresses the pathogenesis of weight changes in patients taking AEDs. The weight gain associated with valproate has been studied most widely. Lower blood glucose in patients taking valproate has been proposed as one mechanism that could lead to obesity. The elevated insulin levels found in women could lead to stimulation of eating through the effect of low glucose on glucose responsive neurons in the medial hypothalamus (52). Valproate could also lower glucose through impaired gluconeogenesis. Valproate reduces carnitine levels, which could impair fatty acid metabolism and gluconeogenesis and increases glucose consumption. According to this hypothesis, the neurons in the medial hypothalamus respond to low glucose levels, reduce the efferent inhibitory output to the lateral hypothalamus, and thereby increase feeding (Figure 1). Hyperinsulinemia has been found in women taking valproate (52), but elevated insulin levels were not found in pubertal and prepubertal girls who gain weight with valproate (58). These results do not support a role for insulin and low glucose in the pathogenesis of valproate obesity in pubertal and prepubertal girls. Valproate could also act to increase appetite and reduce energy expenditure by enhancing GABA-mediated inhibition in the medial hypothalamus. Valproate has been shown to enhance GABA-mediated inhibition, but only at high drug concentrations (68). Valproate also increases whole-brain GABA levels (68). Circulating catecholamines increase in patients taking valproate, and this increase could decrease thermogenesis. Breum et al. (69), however, did not find evidence for greater thermogenesis in their study of patients taking valproate. Short-term clinical studies have not provided support for any of the proposed mechanisms for weight gain in patients taking valproate. No change in appetite or thirst has been found in patients who gain weight with valproate (59,61). Breum et al. (69) did not find any difference in total energy intake or macronutrient selection, energy expenditure, or thyroid hormones. The exact mechanism for weight gain with valproate requires additional study. Verotti et al. (70) found an increase in serum leptin in women who began valproate treatment and subsequently gained weight. Serum leptin increases with increase in weight and number of fat cells; therefore, this result is not surprising. Because leptin-receptor defects in animals are associated with obesity, an alternative explanation might be that these women were predisposed to obesity by a preexisting deficient response to leptin. The baseline leptin levels of women who gained weight with valproate were not different from those of women who did not gain weight with valproate. These data, therefore, would not support a preexisting abnormality in leptin response. The mechanisms of weight gain with gabapentin and vigabatrin could reflect enhancement of GABAmediated inhibition in the medial hypothalamus. As noted in Figure 1, GABA injections in the hypothalamus seem to increase food ingestion. Opposing this explanation, however, is the finding that weight loss, rather than weight gain, occurs with tiagabine, another AED known to increase GABA-mediated inhibition. Similar to the therapeutic effects of AEDs, the regional variations in the effects of different AEDs on GABA may influence their effect on appetite or caloric expenditure. Because carbamazepine is related to tricyclic antidepressants, the ADVERSE EFFECTS OF ANTIEPILEPTIC DRUGS effects of carbamazepine on weight could relate to its effects on noradrenaline or serotonin neurotransmission. Weight loss with felbamate and topiramate could relate to the glutamate-blocking effect or their multiple sites of action to potentiate GABA-mediated responses and block glutamate neurotransmission (7 1). The effect of glutamate antagonists on feeding has been variable, depending on the site tested and the type of glutamate receptor blocked (72-74). Currently, there are insufficient data about the effects of these drugs on the hypothalamus or other areas of the brain that mediate feeding and hunger to know how these drugs reduce weight. These studies of weight change in patients treated with AEDs illustrate how we can begin to better define the risk factors for the development of adverse effects and uncover their mechanisms. These studies have benefited from quantifiable measures and comparisons of patients taking different AEDs as well as comparisons with healthy patients. It is hoped that prospective, comparative studies of other adverse effects will be done in the future. With a better understanding of the circumstances in which each adverse effect occurs, we may be able to determine the mechanisms that mediate the adverse effects and ultimately find ways to prevent them. REFERENCES I , Brodie MJ, Dichter MA. Established antiepileptic drugs. Seizure 1997;6:159-74. 2. Cramer JA, Fisher R, Ben Menachem E, French J, Mattson RH. New antiepileptic drugs: comparison of key clinical trials. Epilepsia 1999;40:590-600, 3. Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N Engl J Med 1985; 313: 145-5 1. 4. Schlienger RG, Oh PI, Knowles SR, Shear NH. Quantifying the costs of serious adverse drug reactions to antieoileotic drugs. Enilepsiu 1998;39(suppl 7):S27-S32. 5. Food and Drug Administration. Record and reports concerning adverse drug experiences on marketed prescription drugs. Washington, DC: Federal Register; October 7, 1997. 6. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf 1999;20109-17. 7. Berkovic SF. Aggravation of generalized epilepsies. Epilepsia 1998;39(suppl 3):SIl-S14. 8. Coppola G, Terraciano AM, Pascotto A. Vigabatrin as add-on therapy in children and adolescents with refractory epilepsy: an open trial. Bruin Dev 1997;19:459-63. 9. Perucca E, Gram L, Avanzini G, Dulac 0. Antiepileptic drugs as a cause of worsening seizures. Epilepsia 1998;39:5-17. 10. Guerrini R, Dravet C, Genton P, et al. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia 1998;39:50812. 11. Elger CE, Bauer J, Scherrmann J, Widman G. Aggravation of focal epileptic seizures by antiepileptic drugs. Epilepsia 1998;39(suppl 3):s15-S I 8. 12. Stecker MM, Kita M. Paradoxical response to valproic acid in a patient with a hypothalamic hamartoma. Ann Pharmacother 1998; 32:1168-72. 13. Jacob PC, Chand RP. Pathological laughter following intravenous sodium valproate. Can J Neurol Sci 1998;25:252-3. 14. Guerrini R, Belmonte A, Genton P. Antiepileptic drug-induced ~I I . S51 worsening of seizures in children. Epilepsia 1998;39(suppl 3):S2s10. 15. Ben Menachem E. Vigabatrin. Epilepsia 1995;36(suppI 2):S95104. 16. Thomas L, Trimble M, Schmitz B, Ring H. Vigabatrin and behaviour disorders: a retrospective survey. Epilepsy Res 1996;25:21-7. 17. Welty TE, Privitera M, Shukla R. Increased seizure frequency associated with felhamate withdrawal in adults. Arch Neurol 1998; 55:641-5. 18. Rowan AJ. Reflections on the treatment of seizures in the elderly population. Neurology 1998;51(suppl 4):S28-S33. 19. Parker BM, Vestal RE. The use of anticonvulsant drugs in the elderly. In: Wyllie E, ed. The treatment of epilepsy: principles and practice. Philadelphia: Lea & Febiger, 1997:747-54. 20. Blackhurn SC, Oliart AD, Garcia Rodriguez LA, Perez GS. Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy 1998;18:1277-83. 21. Brown WM, Aiken SP. Felbamate: clinical and molecular aspects of a unique antiepileptic drug. Crit Rev Neurobiol 1998;12:20522. 22. Schlienger RG, Shapiro LE, Shear NH. Lamotrigine-induced severe cutaneous adverse reactions. Epilepsia 1998;39(suppl7):S22S26. 23 Pellock JM. Treatment of seizures and epilepsy in children and adolescents. Neurology 1998;s I(supp1 4):S8-S14. 24 Bryant AE 111, Dreifuss FE. Valproic acid hepatic fatalities, III: US experience since 1986. Neurology 1996;46:465-9. 25 Appleton RE, Farrell K, Applegarth DA, et al. The high incidence of valproate hepatotoxicity in infants may relate to familial metabolic defects. Can J Neurol Sci 1990;17:145-8. 26. Fromenty B, Pessayre D. Inhibition of mitochondria1 betaoxidation as a mechanism of hepatotoxicity. Pharmacol Ther 199Si67:101-54. 27. Konig SA, Schenk M, Sick C, et al. Fatal liver failure associated with valproate therapy in a patient with Friedreich’s disease: review of valproate hepatotoxicity in adults. Epilepsia 1999;40: 1036-40. 28. Park BK, Pirmohamed M, Kitteringham NR. The role of cytochrome P450 enzymes in hepatic and extrahepatic human drug toxicity. Pharmacol Ther 1995;68:385-424. 29. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol 1994;102:28S-308. 30. Leeder JS. Mechanisms of idiosyncratic hypersensitivity reactions to antiepileptic drugs. Epilepsia 1998;39(suppl7):S8-S16. 31. Pellock JM, Brodie MJ. Felbamate: 1997 update. Epilepsia 1997; 38: 1261-4. 32. Kaufman DW, Kelly JP, Anderson T, Harmon DC, Shapiro S. Evaluation of case reports of aplastic anemia among patients treated with felbamate. Epilepsia 1997;38: 1265-9. 33. Schlienger RG, Shear NH. Antiepileptic drug hypersensitivity syndrome. Epilepsia 1998;39(suppl 7):S3-S7. 34. Guberman AH, Besag FM, Brodie MJ, et al. Lamotrigineassociated rash: riswbenefit considerations in adults and children. Epilepsia 1999;40:985-91. 35. Khafaga YM, Jamshed A, Allam AA, et al. Stevens-Johnson syndrome in patients on phenytoin and cranial radiotherapy. Acta OnC O ~1999;38:111-6. 36. Duncan KO, Tigelaar RE, Bolognia JL. Stevens-Johnson syndrome limited to multiple sites of radiation therapy in a patient receiving phenobarbital. J A m Acad Dermatol 1999;40:493-6. 37. Mamon HJ, Wen PY, Burns AC, Loeffier JS. Allergic skin reactions to anticonvulsant medications in patients receiving cranial radiation therapy. Epilepsia 1999;40:341-4. 38. May RB, Sunder TR. Hematologic manifestations of long-term valproate therapy. Epilepsia 1993;34:1098-101. 39. Brodie MJ. Drug interactions in epilepsy. Epilepsia 1992;33(suppl 1):s13-S22. 40. Tanaka E. Clinically significant pharmacokinetic drug interactions between antiepileptic drugs, J Clin Phnrm Ther 1999;24:87-92. 41. Dreifuss FE, Santilli N, Langer DH, et al. Valproic acid hepatic fatalities: a retrospective review. Neurology 1987;37:379-85. Epilepsiu, Vol. 41, Suppl. 2, 2000 S52 R. S. GREENWOOD 42. Levy RH, Mattson RH, Meldrum BS. Antiepileptic Drugs. 4th ed. New York, NY: Raven Press, 1995. 43. Shorvon SD. The treatment of epilepsy. Oxford, England: Blackwell Science, 1996. 44. Wyllie E. The Treatment of epilepsy: principles and practice. 2nd ed. Baltimore, Md: Williams & Wilkins, 1997. 45. Engel J, Pedley TA. Epilepsy: a comprehensive textbook. Philadelphia, Pa: Lippincott-Raven, 1998. 46. Ben Menachem E, Eadie MJ, Vajda FJE. Antiepileptic drugs: pharmacology and therapeutics. Vol 5. Berlin, Germany: Springer, 1999. 47. Biller J. Iatrogenic neurology. Boston, Ma: Butterworth-Heinemann, 1998. 48. Pijl H, Meinders AE. Bodyweight change as an adverse effect of drug treatment: mechanisms and management. Drug Snf 1996;14: 32942. 49. Considine RV, Sinha MK, Heiman ML, et al. Serum immnnoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996;334:292-5. SO. Carmel PW. Vegetative dysfunctions of the hypothalamus. Acta Neurochir (Wien) 1985;75:113-21. 5 1. Leibowitz SF. Neurochemical-neuroendocrine systems in the brain controlling macronutrient intake and metabolism. Trends Neurosci 1992;15:491-7. 52. lsojarvi JI, Laatikainen TJ, Knip M, et al. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol 1996;39:579-84. 53. National Center for Health Statistics. Healthy People 2000 Review, 1998-1999. Hyattsville, Maryland: Public Health Service, 1999. DHHS Publication (PHS) 99-1 256. 54. Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Engl J Med 1992;327:765-7 1. 55. Isojarvi JI, Rattya J, Myllyla VV et al. Valproate, lamotrigine, and insulin-mediated risks in women with epilepsy. Ann Neurol 1998; 43446-5 1. 56. Morgan JI, Curran T. Calcium and proto-oncogene involvement in the immediate- early response in the nervous system. Ann NYAcad Sci 1989;568:283-90. 57. Novak GP, Maytal J, Alshansky A, et al. Risk of excessive weight gain in epileptic children treated with valproate. J Child Neurol 1999;14:490-5. 58. Rattya J, Vainionpaa L, Knip M, Lanning P, lsojarvi JI. The effects of valproate, carbamazepine, and oxcarbazepine on growth and sexual maturation in girls with epilepsy. Pediatrics 1999;103:58893. 59. Chadwick D. Safety and efficacy of vigabatrin and carbamazepine in newly diagnosed epilepsy: a multicentre randomised doubleblind study. Vigabatrin European Monotherapy Study Group. Lancet 1999;354:13-9. 60. Easter D, O'Bryan-Tear CG, Verity C. Weight gain with valproate or carbamazepine: a reappraisal. Seizure 1997;6: 121-5. 61. Dinesen H, Gram L, Andersen T, Dam M. Weight gain during treatment with valproate. Acta Neurol Scand 1984;70:65-9. 62. Buchanan N. The use of lamotrigine in juvenile myoclonic epilepsy. Seizure 1996;5:149-51. 63. Lamp1 Y, Eshel Y, Rapaport A, Sarova-Pinhas I. Weight gain, increased appetite, and excessive food intake induced by carbamazepine. Clin Neuropharmacol 1991;14:251-5. 64. Carmant L, Holmes GL, Sawyer S, et al. Efficacy of felbamate in therapy for partial epilepsy in children. J Pediatr 1994; lZ:481-6. 65. Bergen DC, Ristanovic RK, Waicosky K, Kanner A, Hoeppner TJ. Weight loss in patients taking felbamate. Clin Neuropharmacol 1995;18~23-7. 66. Tartara A, Sartori I, Manni R, et al. Efficacy and safety of topiramate in refractory epilepsy: a long-term prospective trial. Ital J Neurol Sci 1996;17:429-32. 67. Shorvon SD. Safety of topiramate: adverse events and relationships to dosing. Epilepsia 1996;37(suppl 2):S18-S22. 68. Thomas AB, Matthews GC, Ferrendelli JA. Mechanisms of action of antiepileptic drugs. In: Wyllie E, ed. The treatment of epilepsy: principles and practice. Philadelphia: Lea & Febiger, 1997:74754. 69. Breum L, Astrup A, Gram L, et al. Metabolic changes during treatment with valproate in humans: implication for untoward weight gain. Metabolism 1992;41:666-70. 70. Verrotti A, Basciani F, Morresi S, et al. Serum leptin changes in epileptic patients who gain weight after therapy with valproic acid. Neurology 1999;53:230-2. 71. Meldrum BS. Update on the mechanism of action of antiepileptic drugs. Epilepsia 1996;37(suppl 6):S4-S11. 72. Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 1999;19: 11040-8. 73. Khan AM, Curras MC, Dao J, et al. Lateral hypothalamic NMDA receptor subunits NR2A and/or NR2B mediate eating: immunochemicallbehavioral evidence. A m J Physiol 1999;276:R880R891. 74. Stratford TR, Swanson CJ, Kelley A. Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res 1998;93:43-SO. 75. Leber PD. Hazards of' inference: the active control investigation. Epilepsia 1989;3O(suppl l):S57-S63. 76. Shorvon S, Stefan H. Overview of the safety of newer antiepileptic drugs. Epilepsia 1997;38(suppl l):S45-S51. 77. Chiron C, Dulac 0, Beaumont D, et al. Therapeutic trial of vigabatrin in refractory infantile spasms. J Child Neurol 1991;(suppl 2):S52-S59. 78. Corman CL, Leung NM, Guherman AH. Weight gain in epileptic patients during treatment with valproic acid: a retrospective study. Can J Neurol Sci 1997;24:2404. 79. FelbatoP [package insert]. Abbott Park, Ill: Abbott Laboratories; 2000. 80. Dodson WE. Felbamate in the treatment of Lennox-Gastaut syndrome: results of a 12-month open-label study following a randomized clinical trial. Epilepsia 1993;34(suppl 7):s 1X-S24. 81. Sachdeo RC. Topiramate: clinical profile in epilepsy. Clin Pharmacokinet 1998;34:33546. 82. Leppik IE, Dreifuss FE, Pledger GW, et al. Felbamate for partial seizures: results of a controlled clinical trial. Neurology 1991;41: 1785-9. 83. Glauser TA. Topiramate. Semin Pediatr Neurol 1997;4:3442. 84. Biton V, Moutouris GD, Ritter F, et al. A randomized, placebocontrolled study of topiramate in primary generalized tonic-clonic seizures. Topiramate YTC Study Group. Neurology 1999;52: 1330-7.