* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download DRUG ELIMINATION
Survey
Document related concepts
Transcript
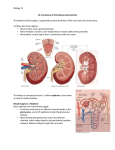
LECTURE 5 PHARMACOLOGY DRUG ELIMINATION Drug Elimination Sites of Action Absorption Unbound Drug Metabolism Tissue Depots Bound Drug Excretion Drug Elimination Direct filtration & elimination through kidneys Drug Metabolism by liver to inactive product Active secretion by kidneys Metabolism by liver to active product D r u g E l i m i n a t i o n Drug Elimination Direct filtration & elimination through kidneys Drug Active secretion by kidneys D r u g E l i m i n a t i o n Drug Elimination • MAJOR ROUTES – Liver – Kidneys • Minor routes of elimination – Lungs (Volatile general anesthetics) – Sweat – Saliva – Mother’s milk NEED FOR METABOLISM KIDNEY Is Major Site of Drug Elimination Water Soluble Filtration Lipid Soluble in Glomerulus LIVER KIDNEY Lipid soluble drugs are reabsorbed!!! Secretion of organic acids and bases Back diffusion is dependent on pH of tubular fluid & lipid solubility of drug Excretion in Urine The Kidney Arterial supply Glomerulus (1.3 L/min) Collecting tubule Proximal tubule Distal tubule Venous return Active secretion Reabsorption Urine e.g., gentamicin, cephalexin (1.5 L/day) Loop of Henle Blood Flow in the Kidney Is Important • Renal blood flow is ~25% of cardiac output – 1.3 L/min • Renal plasma flow is 50% of renal blood flow – 650 ml/min • Glomerular filtration rate (GFR) is 20% of plasma flow – – – – 130 ml/min In 24 hr, 185-190 Liters are filtered by the glomerulus 24 hr urine output is 1.5-1.7 Liters More than 99% of glomerular filtrate volume must be reabsorbed • BUT water reabsorption does NOT equal solute reabsorption Drug Excretion in the Kidney • Two Components – Glomerular filtration • Passive (no energy) • Clears free drug only • 130 ml/min – Tubular secretion • Active (requires energy) • Can clear 90-100% of drug flowing through kidney – 650 ml/min (5X glomerular filtration) Glomerular Filtration • The Glomerulus – Filters 100% of blood supply – Filters everything <40 kDa • Plasma & small proteins • Glomerular Filtration Rate – ~130 ml/min – Measured by inulin or creatinine – Creatinine clearance is a measure of kidney health • Used to adjust drug dosage if needed Arterial supply (130 ml/min) Tubular Secretion • Energy-dependent transport (secretion) – Occurs from blood into • Proximal tubule • Distal tubule • Loop of Henle – Can clear blood of 100 of drug passing through kidney • Separate transport systems for – Weak organic bases (WOBs) – Weak organic acids (WOAs) • Most drugs and metabolites are WOAs • Probenecid is a substrate for WOA transporters – Its administration inhibits secretion of many drugs Tubular Secretion • Drugs are NOT normal substrates – Drugs compete with other drugs – BUT Drugs compete with endogenous metabolites – In particular metabolic acids • Sulfate • Phosphate • Glucuronate (sugar acids) – Can cause electrolyte disturbances Tubular Reabsorption • Mostly by passive non-ionic diffusion • Non-ionized forms of drug reabsorbed – 60% in proximal tubule – Uric acid (urate) is the exception (active) THEREFORE • Acid & base forms of drugs are secreted • Lipophilic (non-ionic) forms of drugs are reabsorbed CONSEQUENTLY • Blood and urine pH affect drug elimination CLEARANCE • A very important concept for drug use • Clearance (Cl) is the VOLUME of fluid (plasma) “cleared” (freed) of drug per unit time • Clearance of most drugs is a first order process – A constant fraction of drug is cleared per unit time • A fraction is NOT a concentration • Therefore, first order clearance is independent of drug concentration CLEARANCE • Clearance is independent of the method and route of clearance – – – – – Hepatic clearance Renal clearance Lung (inhalational) clearance Saliva Mother’s milk Therapeutic Implications of Clearance • Highly ionized drugs tend to be rapidly cleared – Minimal tubular reabsorption since only non-ionized drug is reabsorbed • Alkalinizing urinary pH with Na bicarbonate can accelerate clearance of WOAs – Salicylate and barbiturates • Acidifying urinary pH with arginine hydrochloride can accelerate clearance of WOBs – Amphetamines Therapeutic Implications of Clearance • Drug forms that are quite lipid soluble at the pH of the urine (5.5) are readily reabsorbed – Maximal tubular reabsorption since non-ionized drug is reabsorbed • Increasing osmolarity of urine (mannitol) may increase elimination of a lipophilic drug Therapeutic Implications of Clearance • Tubular secretion of a drug may be inhibited by another drug by competition for the transporter – Probenecid competes with penicillins • Thus prolongs action of antibiotic – Probenecid competes with some diuretics (furosemide) and thus may prevent diuretic access to the tubule which is where they act • Decreases effect of diuretic Therapeutic Implications of Clearance • Drug clearance is decreased by renal disease – Measured by creatinine clearance – Caused by • Decreased renal blood flow • Glomerular tubular damage • Tubular nephropathy • Drug clearance is greater in an adult than in – Children (immaturity of kidney function) – Elderly (decreased renal function – Alcoholics Drug Metabolism • Most metabolic products are less pharmacologically active Important exceptions: • Where the metabolite is more active (Prodrugs, e.g. Erythromycin-succinate (less irritation of GI) --> Erythromycin) • Where the metabolite is toxic (acetaminophen) • Where the metabolite is carcinogenic • Close relationship between the biotransformation of drugs and normal biochemical processes occurring in the body: – Metabolism of drugs involves many pathways associated with the synthesis of endogenous substrates such as steroid hormones, cholesterol and bile acids – Many of the enzymes involved in drug metabolism are principally designed for the metabolism of endogenous compounds – These enzymes metabolize drugs only because the drugs resemble the natural compound Phases of Drug Metabolism • Phase I Reactions – Convert parent compound into a more polar (=hydrophilic) metabolite by adding or unmasking functional groups (-OH, -SH, -NH2, -COOH, etc.) – Often these metabolites are inactive – May be sufficiently polar to be excreted readily • Phase II Reactions – Conjugation with endogenous substrate to further increase aqueous solubility – Conjugation with glucoronide, sulfate, acetate, amino acid – Phase I usually precede phase II reactions Liver is principal site of drug metabolism: – Other sites include the gut, lungs, skin and kidneys – For orally administered compounds, there is the “First Pass Effect” • • • • Intestinal metabolism Liver metabolism Enterohepatic recycling Gut microorganisms - glucuronidases Drug Metabolism - Phase I • Phase I Reactions – – – – – – – – – – – Oxidation Reduction Hydrolytic cleavage Alkylation (Methylation) Dealkylation Ring cyclization N-carboxylation Dimerization Transamidation Isomerization Decarboxylation Drug Metabolism - Oxidation Two types of oxidation reactions: – Oxygen is incorporated into the drug molecule (e.g. hydroxylation) – Oxidation causes the loss of part of the drug molecule (e.g. oxidative deimination, dealkylation) Microsomal Mixed Function Oxidases (MFOs) • “Microsomes” form in vitro after cell homogenization and fractionation of ER – Rough microsomes are primarily associated with protein synthesis – Smooth microsomes contain a class of oxidative enzymes called • “Mixed Function Oxidases” or “Monooxygenases” – These enzymes require a reducing agent (NADPH) and molecular oxygen (one oxygen atom appearing in the product and the other in the form of water) Drug Metabolism - Oxidation • MFO consists of two enzymes: – Flavoprotein, NADPH-cytochrome c reductase • One mole of this enzyme contains one mole each of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) • Enzyme is also called NADPH-cytochrome P450 reductase – Cytochrome P450 • named based on its light absorption at 450 nm when complexed with carbon monoxide • is a hemoprotein containing an iron atom which can alternate between the ferrous (Fe++) and ferric (Fe+++) states • Electron acceptor • Serves as terminal oxidase • its relative abundance compared to NADPH-cytochrome P450 reductase makes it the rate-limiting step in the oxidation reactions Cytochrome P450 At least 57 different isozymes in humans, over 7700 forms in Nature isozyme-catalytically and structurally similar but genetically distinct enzymes-different genes and amino acid sequences Different isozymes have different substrate specificities Individuals have several alleles for P450’s and differ in which isozymes they have Since individuals have different combinations of P450’s, they differ in their response to specific drugs A subset of cytochrome P450’s can be induced, so that more is expressed upon exposure to a compound. Because of the number of different isozymes and their different substrates and inhibitors, the metabolism of a drug can be altered if an individual takes a second drug. Some substrates of cytochrome P450 isozymes 1A2 2B6 2C8 2C19 2C9 2D6 2E1 3A4,5,7 amitriptyline caffeine clomipr amine clozapine cyclobenzaprine estradiol fluvoxamine haloperidol imipramine NDeMe mexilletine naproxen olanzapine ondansetron phenacetin acetaminophen propranolol riluzole ropivacaine tacrine theophylline tizanidine verapamil bupropion cyclophosphamide efavirenz ifosfamide methadone paclitaxel torsemide amodiaquine cerivastatin repaglinide Proton Pump Inhibitors: lansoprazole omeprazole pantoprazole rabeprazole ibuprofen meloxicam S-naproxen piroxicam suprofen Beta Blockers: Anesthetics: enflurane halothane isoflurane methoxyflurane sevoflurane erythromycin telithromycin Anti-epileptics: diazepam phenytoin(O) S-mephenytoin phenobarbitone amitriptyline carisoprodol citalopram chloramphenicol clomipr amine cyclophosphamide hexobarbital imipramine indomethacin R-mephobarbital Oral Hypoglycemic Agents: tolbutamide glipizide Angiotensin II Blockers: losartan irbesartan Sulfonylureas: glyburide/ glibenclamide glimepiride tolbutamide amitriptyline clomipr amine desipramine imipramine paroxetine Benzodiazepines: cyclosporine tacrolimus (FK506) haloperidol HIV Antivirals alprenolol amphetamine aripiprazole atomoxetine bufuralol chlorpheniramine chlorpromazine codeine Antihistamines: astemizole chlorpheniramine terfenadine fluvoxamine lidocaine metoclopramide methoxyamphetam ine Calcium Channel Blockers HMG CoA Reductase Inhibitors: atorvastatin cerivastatin Drug Metabolism - Phase II • Conjugation reactions – Glucuronidation by UDP-Glucuronosyltransferase: (on -OH, -COOH, -NH2, -SH groups) – Sulfation by Sulfotransferase: (on -NH2, -SO2NH2, -OH groups) – Acetylation by acetyltransferase: (on -NH2, -SO2NH2, -OH groups) – Amino acid conjugation (on -COOH groups) – Glutathione conjugation by Glutathione-S-transferase: (to epoxides or organic halides) – Fatty acid conjugation (on -OH groups) – Condensation reactions Drug Metabolism - Glucuronidation • Glucuronidation ( = conjugation to a-d-glucuronic acid) – Quantitatively the most important phase II pathway for drugs and endogenous compounds – Products are often excreted in the bile. – Enterohepatic recycling may occur due to gut glucuronidases – Requires enzyme UDP-glucuronosyltransferase (UGT): • Genetic family of enzymes – Metabolizes a broad range of structurally diverse endogenous and exogenous compounds – Structurally related family with approximately 16 isoforms in man Drug Metabolism - Glucuronidation • Glucuronidation – requires creation of high energy intermediate: UDP-Glucuronic Acid: NEXT LECTURE AUTONOMIC NERVOUS SYSTEM APPLIED PHARMACOLOGY THANK YOU



































![[4-20-14]](http://s1.studyres.com/store/data/003097962_1-ebde125da461f4ec8842add52a5c4386-150x150.png)