* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download chapter 52 - Lange Textbooks
Neonatal infection wikipedia , lookup
Gastroenteritis wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Childhood immunizations in the United States wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Kawasaki disease wikipedia , lookup
Ankylosing spondylitis wikipedia , lookup
Infection control wikipedia , lookup
Sarcocystis wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Behçet's disease wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Germ theory of disease wikipedia , lookup
Globalization and disease wikipedia , lookup
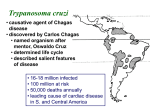
CHAPTER 52 Flagellates Although a number of flagellate genera parasitize humans, only four, Trichomonas, Giardia, Leishmania, and Trypanosoma, commonly induce disease. Trichomonas and Giardia are noninvasive organisms that inhabit the lumina of the genitourinary or gastrointestinal tract and are spread without benefit of an intermediate host. Disease is of low morbidity and cosmopolitan distribution. Leishmania and Trypanosoma, on the other hand, are invasive blood and tissue parasites that produce highly morbid, frequently lethal diseases. These hemoflagellates require an intermediate insect host for their transmission. As a result, their associated disease states are limited to the semitropical and tropical niches of these intermediate hosts. General Features 1. Multiply by binary fission, and move about by means of cytoplasmic organelles of locomotion 2. Flagellum arises from an intracellular focus known as a blepharoplast, extends to the cell wall as a filamentous axoneme, and continues extracellularly as the free flagellum 3. In some species, the blepharoplast is paired with a second cytoplasmic structure known as a parabasal body 4. In many flagellates the axoneme lifts a segment of external wall into a longitudinal undulating 5. Elongated or oval in shape and often possess a rudimentary cytostome (mouth aperture) and organelles such as sucking discs or axostyles, which help them maintain their intraluminal position 6. All can be cultivated on artificial media. 7. Some luminal flagellates, most notably T. vaginalis, possess only a trophozoite stage 8. Most, including G. lamblia, possess both trophozoite and cyst forms Trichomonas vaginalis I. PARASITOLOGY 1. Three Trichomonas species have similar morphology but only T. vaginalis is an established pathogen 2. The T. vaginalis trophozoite is oval with a single, elongated nucleus , small cytostome and five flagella 3. Protruding axostyle may mediate attachment 4. Cultivable in vitro 5. Lacks cyst form but survives a few hours outside host II. TRICHOMONIASIS A. EPIDEMIOLOGY 1. Transmission usually sexual 2. Prevalence linked to sexual activity 3. Nonvenereal transmission on shared washcloths is uncommon B. PATHOGENESIS 1. Parasite damages epithelial cells on contact 2. Parasitic glycoprotein correlates with clinical manifestations 3. Changes in hormonal and pH environment of the vagina modulate severity of pathologic changes III. TRICHOMONIASIS: CLINICAL ASPECTS A. MANIFESTATIONS 1. Chronic vaginitis lasting weeks to months 2. Discharge, vulvar itching, burning, dyspareunia, dysuria, and a disagreeable odor are the symptoms 3. Discharge is typically thin, yellow, and frothy in character 4. Urethral and prostatic infection in men usually asymptomatic B. DIAGNOSIS 1. Wet mount examination of vaginal discharge for motile trophozoites is sufficient in most symptomatic cases 2. In men, urethral exudate or urine sediment after prostate massage may be used C. TREATMENT 1. Metronidazole cures 95% of cases 2. Simultaneous treatment of sexual partners may minimize recurrent infections Giardia lamblia I. PARASITOLOGY 1. Both trophozoite and cyst stages present 2. Sting-ray–shaped trophozoite’s two nuclei and central parabasal bodies give the appearance of a face with two bespectacled eyes and a crooked mouth 3. Move about duodenum and jejunum with tumbling motility 4. Using a large ventral sucker trophs attach themselves to the brush border of the intestinal epithelium 5. Oval cystic forms develop in colon 6. Resistant cysts transmitted from host to host 7. Wide distribution in animal kingdom II. GIARDIASIS A. EPIDEMIOLOGY 1. Direct person-to-person transmission facilitated by poor hygiene and IgA deficiency 2. High attack rates in day-care centers 3. Giardiasis frequent among male homosexuals 4. Water- or food-borne traveler’s diarrhea lasts for weeks 5. Beavers and other mammals possible sources B. PATHOGENESIS 1. Disease manifestations related to intestinal malabsorption, particularly of fat and carbohydrates 2. Jejunal biopsies sometimes reveal a flattening of the microvilli and an inflammatory infiltrate 3. Basis for malabsorption and jejunal pathology remains uncertain C. IMMUNITY 1. Predisposing factors include hypochlorhydria and immunocompromise 2. Although reinfection is common rarity in older adults, suggests protective immunity, albeit incomplete, does develop in humans III. GIARDIASIS: CLINICAL ASPECTS A. MANIFESTATIONS 1. 2. 3. 4. Subclinical infections common in endemic areas Diarrhea, cramping, flatus, and greasy stools Subacute and chronic infections with weight loss in adults Lactose intolerance may persist B. DIAGNOSIS 1. Demonstration of trophozoites and cysts in stool or duodenal aspirates is diagnostic 2. EIAs detect Giardia antigen in stool C. TREATMENT 1. Four drugs quinacrine hydrochloride, metronidazole, furazolidone, and paromomycin are effective 2. Close contacts should be examined D. PREVENTION 1. Hikers should avoid ingestion of untreated surface water, even in remote areas 2. Disinfection can be accomplished with halogen tablets yielding concentrations higher than that generally achieved in municipal water systems BLOOD AND TISSUE FLAGELLATES 1. Two of the many genera of hemoflagellates are pathogenic to humans, Leishmania and Trypanosoma 2. Life cycle includes insect host stage 3. Promastigote and epimastigote forms in insects 4. Trypomastigote and amastigote forms in humans 5. The flagellated forms move in a spiral fashion, and all reproduce by longitudinal binary fission Leishmania I. PARASITOLOGY 1. Leishmania species are obligate intracellular parasites of mammals. Several strains can infect humans 2. Species are morphologically similar 3. Differentiation requires isoenzyme analysis and molecular features A. DISEASE TRANSMISSION 1. 2. 3. 4. 5. 6. Over 20 million people worldwide suffer from Cutaneous ulcer or visceral infection (kala azar) the primary diseases All four groups transmitted by nocturnally feeding sandflies Complement activation mediates attachment to macrophages Intracellular survival by inhibiting macrophage killing mechanisms Amastigotes released from macrophages can infect feeding sandfly 7. In localized cutaneous disease, cellular immune responses produce spontaneous cure 8. Mucocutaneous metastases in L. braziliensis infections 9. Lack of cellular immune response in disseminated and chronic infections II. LOCALIZED CUTANEOUS LEISHMANIASIS A. EPIDEMIOLOGY 1. The disease is a zoonotic infection of tropical and subtropical rodents 2. Particularly common in areas of Central Asia, the Indian subcontinent, Middle East, Africa, the Mediterranean littoral, and Central and South America 3. Geographic distribution related to human and rodent reservoirs 4. Canine reservoir in urban disease B. MANIFESTATIONS 1. Lesions usually appear on the extremities or face weeks to months after the bite of the sandfly 2. Appear as pruritic papules and evolve to chronic, self-limiting skin ulceration 3. Strain-specific immunity C. DIAGNOSIS AND TREATMENT 1. Diagnosis is made on clinical grounds and confirmed by the demonstration of the organism in the advancing edge of the ulcer 2. Demonstration of Leishman–Donovan bodies or culture from tissue biopsy 3. Can also be cultured in liquid media. 4. Small, cosmetically minor lesions may be carefully followed without treatment 5. Pentavalent antimonial agents and liposomal amphotericin B have proved to be effective for more consequential lesions III. MUCOCUTANEOUS LEISHMANIASIS A. EPIDEMIOLOGY 1. L. braziliensis causes a natural infection in the large forest rodents of tropical Latin America 2. Sandflies transmit the infection to humans B. MANIFESTATIONS 1. Primary lesion metastasizes to oral and nasal areas 2. progressively enlarges, often producing large vegetating lesions 3. Fever, anemia, weight loss, and secondary bacterial infections are common C. DIAGNOSIS AND TREATMENT Detection of organisms as with cutaneous leishmaniasis Treatment is with the agents used for kala azar IV. DISSEMINATED VISCERAL LEISHMANIASIS (KALA AZAR) A. EPIDEMIOLOGY 1. Kala azar, which is caused by L. donovani, occurs in the tropical and subtropical areas of every continent except Australia 2. Transmission is carried out by anthropophilic species of sandflies 3. Marked geographic differences in reservoirs and disease severity B. PATHOGENESIS 1. Parasites disseminate in the bloodstream to spleen, liver, bone marrow, lymph nodes, skin, and small intestine 2. Invade and multiply in macrophages of reticuloendothelial system V. KALA AZAR: Clinical Aspects A. MANIFESTATIONS 1. Delayed onset; recurrent fever; chronic disease; diarrhea 2. Physical findings include enlarged lymph nodes and liver, massively enlarged spleen, and edema 3. Severe systemic manifestations 4. Immune complex glomerulonephritis B. DIAGNOSIS AND TREATMENT 1. Demonstration of Leishman–Donovan bodies or culture 2. Treatment with pentavalent antimonial 3. Resistant cases are treated with the more toxic pentamidine, amphotericin B, or liposomal amphotericin B 4. Up to 90% mortality without treatment African Trypanosoma I. PARASITOLOGY 1. Three recognized subspecies of T. brucei 2. Epimastigote and trypomastigote forms develop in tsetse fly 3. Infectious trypomastigote form injected into the bloodstream of mammalian host from fly's saliva 4. Antigenic variation of glycoprotein coat of trypomastigotes is due to shifting expression of preexisting genes II. AFRICAN TRYPANOSOMIASIS (SLEEPING SICKNESS) A. EPIDEMIOLOGY 1. Tsetse fly confined to central Africa 2. Humans major reservoir of West African sleeping sickness; chronicity ensures maintenance 3. Savanna antelopes are reservoirs of East African trypanosomiasis; humans infected incidentally B. PATHOGENESIS 1. 2. 3. 4. 5. Local chancre at site of inoculation and lymphadenitis Intermittent parasitemia with antigenic shifts Parasites localize in blood vessels of heart and CNS with local vasculitis High levels of IgM include specific and nonspecific antibodies Immune complexes may cause anemia and vasculitis III. AFRICAN TRYPANOSOMIASIS (SLEEPING SICKNESS): CLINICAL ASPECTS A. MANIFESTATIONS 1. Raised red papule on exposed surface 2. Parasitemic manifestations 2–3 weeks later 3. In the Rhodesian form of disease, myocarditis and CNS involvement begin within 3 to 6 weeks 4. Late CNS involvement: Spontaneous activity diminishes, attention wavers, speech grows indistinct, tremors develop, and seizures with transient bouts of paralysis occur 5. In the terminal stage the patient lapses into a final coma B. DIAGNOSIS 1. Trypomastigotes sought in lymph node aspirates, blood, and cerebrospinal fluid 2. Animal inoculation may be required in Rhodesian disease C. TREATMENT 1. Selection of drugs dependent on whether CNS is involved 2. Most effective CNS agent is a highly toxic arsenical, melarsoprol 3. If the CNS is not yet involved, less toxic agents, such as suramin, pentamidine, or eflornithine, can be used 4. Without CNS involvement, recovery often complete D. PREVENTION 1. A degree of personal protection can be achieved with insect repellents and protective clothing 2. Neither vector or reservoir control has been successful American Trypanosoma I. PARASITOLOGY 1. The trypomastigotes of Trypanosoma cruzi closely resemble those of T. brucei 2. Mammalian cycle with nondividing extracellular trypomastigotes and dividing intracellular amastigotes 3. Invertebrate cycle produces trypomastigotes in bug 4. Reduviid bug may remain infectious for up to 2 years II. AMERICAN TRYPANOSOMIASIS (CHAGAS' DISEASE) A. EPIDEMIOLOGY 1. Chagas' disease affects 16 to 18 million people in a geographic area extending from Central America to southern Argentina 2. "Kissing bug" feeds at night in rural areas 3. Other wild and domestic animal reservoirs amplify transmission B. PATHOGENESIS 1. 2. 3. 4. 5. Local chancre at site of inoculation Entry to mesenchymal cells facilitated by fibronectin binding surface protein Pore-forming protein aids escape from phagolysosome Pseudocysts formed from cytoplasmic multiplication in host cells Damage to heart may have immune mechanism 6. Ganglionic and smooth muscle cells lost in digestive tract III. AMERICAN TRYPANOSOMIASIS (CHAGAS' DISEASE): CLINICAL ASPECTS A. MANIFESTATIONS 1. Most infections asymptomatic; acute disease usually in children 2. Myocardial injury indicated by tachycardia and electrocardiographic changes 3. Chronic cardiomyopathy in adults leads to heart block and/or congestive heart failure 4. Dilatation of esophagus and colon seen in southern latitudes B. DIAGNOSIS 1. Demonstration of trypomastigotes in peripheral blood 2. Xenodiagnosis involves allowing bugs to feed 3. Organisms difficult to recover in chronic disease C. TREATMENT 1. Nifurtimox and benznidazole, effectively reduce the severity of acute disease but appear to be ineffective in chronic infections 2. Allopurinol is capable of suppressing parasitemia D. PREVENTION 1. Control of reduviid bugs in rural homes most important measure 2. Transfusion-induced disease controlled by the addition of gentian violet to blood packs or by screening potential donors serologically for Chagas' disease