* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 2015 Ketamine Rescheduling
Compounding wikipedia , lookup
Electronic prescribing wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Prescription costs wikipedia , lookup
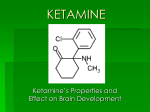
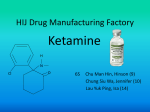
Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, rm. 1061 Rockville, MD 20852 October 13, 2015 Re: Docket No. FDA-2015-N-0045 for International Drug Scheduling; Convention on Psychotropic Substances; Single Convention on Narcotic Drugs; Ketamine; Phenazepam; Etizolam; 1-cyclohexyl-4-(1,2-diphenylethyl)-piperazine (MT-45); N-(1-Phenethylpiperidin-4yl)-N-phenylacetamide (Acetylfentanyl); α-Pyrrolidinovalerophenone (α-PVP); 4Fluoroamphetamine (4-FA); para-Methyl-4-methylaminorex (4,4’-DMAR); paraMethoxymethylamphetamine (PMMA); 2-(ethylamino)-2-(3-methoxyphenyl)-cyclohexanone (Methoxetamine or MXE); Request for Comments To Whom It May Concern: The American Association of Zoo Veterinarians (AAZV) strongly objects to any change in the international regulation of ketamine that would result in this drug being more difficult, if not impossible, to obtain by licensed veterinarians for the authorized treatment of animals. The AAZV is the professional association for veterinarians and institutions that focus on the medical care of zoo and wildlife species. Our over 1000 AAZV members work in clinical zoo medical practices, diagnostic, reproductive and pathological laboratories, pharmaceutical companies, academia, private veterinary practices, and a wide range of governmental health and wildlife management agencies throughout the world. In the United States, ketamine is a Schedule III drug under the Controlled Substance Act, with existing strict regulations to prevent illegal use. While we understand ketamine is not controlled internationally under either the Psychotropic Convention or the Single Convention on Narcotic Drugs, our Association is very concerned for the health and welfare of AAZV member veterinary patients if international regulations elevate the schedule placement of ketamine such that it cannot be accessed by veterinarians. Ketamine plays a key role in veterinary anesthesia for a vast range of species, and is absolutely critical for the safe immobilization and handling of exotic and endangered species in our care. Ketamine has documented minimal physiological effects on the cardiovascular system making it a safe anesthetic choice in aged, ill, or otherwise debilitated veterinary patients. Ketamine is a safe and effective agent when used alone and works well in synergistic combinations with other anesthetic and analgesic agents. There is no ketamine equivalent or substitute for anesthetizing and providing pain relief for our patients. It is essential for the safe handling and manipulation of zoo and wildlife species, the performance of health evaluations, diagnostic and therapeutic procedures, surgical induction, post-surgical evaluations, and the provision of routine medical care in the daily work of AAZV members. The inability to obtain ketamine would be detrimental to the welfare, care, and safety of a wide variety of zoo and wildlife patients, seriously hindering the proper care and management of captive and free-ranging species. Elevating ketamine to a Schedule I drug is not needed and would result in unacceptable negative impacts on animal health and welfare by removing a key component of veterinary anesthesia. By definition, a Schedule I drug means “the drug or other substance has a high potential for abuse; the drug or other substance has no currently accepted medical use in treatment in the United States; and there is a lack of accepted safety for use of the drug or other substance under medical supervision.” Ketamine is already appropriately regulated under Schedule III of the Controlled Substances Act (CSA) (21 CFR & 1308.13(c) (7)). Given the approved medical uses and safe applications of ketamine products in the United States, classification to Schedule I is contrary to the CSA and Drug Enforcement Administration regulations. The regulation and scheduling of drugs, whether pursuant to United States law or International treaty, should maintain a balance between regulations that prevent or deter abuse and maintaining availability of substances for medical purposes. The United States placement of ketamine as a Schedule III controlled drug provides a good example of that appropriate balance. As advocates for our unique and endangered patients, we thank you for the opportunity to provide the FDA with these comments ahead of the World Health Organization’s 36th Expert Committee on Drug Dependence (ECDD), which will meet in Geneva Nov. 16-20, 2015. Sincerely, Kelly Helmick, DVM, MS, DACZM President, American Association of Zoo Veterinarians References Zoo Animal and Wildlife Immobilization and Anesthesia, 2nd edition. Editors West, Heard and Caulkett. Wiley-Blackwell Printing, 2014. ISBN: 978-0-8138-1183-3. Fowler’s Zoo and Wild Animal Medicine., 8th edition. Editors Fowler and Miller. Saunders Printing, 2014. ISBN: 9781455773978. Code of Federal Regulations Title 21- Food and Drugs. Section 1308.13-Schedule III (21 CFR & 1308.13) 1 April 2012. Code of Federal Regulations Title 21 – Food and Drugs. Section 1308.812(b)(1)-Schedule I (21 CFR & 812(b)(1). 1 April 2012.