* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download New Patient
Survey
Document related concepts
Transcript
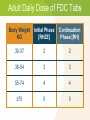
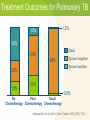
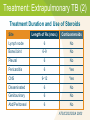
Module 7A – March 2010 Treatment of Tuberculosis: New Case Project Partners Funded by the Health Resources and Services Administration (HRSA) Module Overview Drugs and treatment standards Standard new case regimens and dosages Treatment of EPTB and in special situations International Standards 7, 8 & 10 • • • • Smear-negative PTB Pregnancy Liver disorders Renal disease Learning Objectives At the end of this presentation, participants will be able to: Describe the drug regimens used to treat new TB cases (pulmonary and extrapulmonary) Identify treatment and management approaches in special situations (e.g., smear-negative PTB, pregnancy, presence of co-morbidities) Describe the essential monitoring based on site of disease, bacteriological status, and in special populations First Line Anti-Tuberculosis Drugs Isoniazid (INH, H) Rifampicin (RIF, R) Pyrazinamide (PZA, Z) Ethambutol (EMB, E) Streptomycin (SM, S) Standards for Treatment Standard 7: Public Health Effects of Treatment Practitioners assume an important public health responsibility in ensuring both appropriate treatment regimens and assessment of treatment adherence for their patients. Effect of Treatment on Public Health Why is TB Treatment a Public Health Measure? Providing the patient with an effective treatment that kills the organisms will rapidly reduce and ultimately eliminate the bacillary population in respiratory secretions, thus reducing the potential for transmission Effective multiple-drug treatment, using fixed-dose combination tablets, greatly reduces the risk of resistant organisms emerging Effective treatment decreases the duration and severity of illness and reduces the risk of death Effect of Treatment on Public Health (2) Pulmonary TB cases/100,000 Effects of Treatment on the Incidence of Tuberculosis in Peru 220 DOTS 1990 200 case finding 180 160 140 120 100 PTB falling at 6%/yr 1980 1985 1990 1995 2000 Standard 8: Treatment New Cases All patients (including those with HIV infection) who have not been treated previously should receive an internationally accepted first-line treatment regimen using drugs of known bioavailability The initial phase should consist of 2 months of isoniazid, rifampicin, pyrazinamide and ethambutol The continuation phase should consist of isoniazid and rifampicin given for four months Antituberculosis Drug Activity Drug Early bactericidal activity Preventing drug resistance Sterilizing activity Isoniazid ++++ +++ ++ Rifampicin ++ +++ ++++ Pyrazinamide + ++ - +++ + ++ +++ + Ethambutol Highest ++++ High +++ Intermediate ++ Low + Microbiological Goals - Anti-TB Drugs Microbiological Goals of Antituberculosis Chemotherapy Kill tubercle bacilli rapidly (early bactericidal effect) Prevent the emergence of drug resistance Eliminate persistent bacilli to prevent relapse (sterilizing effect) Adequate TB treatment requires: An appropriate combination of anti-TB medications to prevent resistance Correctly prescribed dosage Taken regularly by the patient Treatment for a sufficient period of time to prevent relapse All doses directly observed (DOT) Never add a single drug to a failing regimen Treatment Effect on Bacillary Population Mixed population (susceptible and resistant) INH resistant bacilli Log cfu Emergence of INH resistant strain because of ineffective treatment (INH monotherapy) Effective multi-drug therapy 0 2 4 6 8 10 12 14 Weeks 16 18 20 22 24 Unintended Monotherapy and Resistance Months of Rx 0 5 7 9 Smear + + + + Culture + + + + INH R* R R R RIF S* R R R EMB S* S S R INH RIF EMB Susceptibility * Results not known to clinician Standard Regimens by Patient Group New Patient – factors influencing regimen: • Presumed to have drug susceptible TB • Patient also HIV co-infected • Setting known to have high prevalence of INH resistance in new patients Previously Treated Patient – regimen is based on: • Availability of drug sensitivity testing • Likelihood of multi-drug resistance (MDR) Benefits of Standard Regimens Reduces errors in prescriptions Facilitates estimates of drug need, purchasing, distribution and monitoring Facilitates staff training Reduces cost Facilitates regular drug supply Makes outcome evaluation convenient and comparable Treatment Recommendations New Patient Groups Presumed or known drug susceptible TB treatment regimens Initial phase (2 mos.) Continuation phase Optimal HRZEa Optimal HR, 4 mos. Acceptable HRZEa or HRZEa, b, 3x/wk Acceptable HR 3x/wk, 4 mos. H = Isoniazid; R = Rifampicin; Z = Pyrazinamide; E = Ethambutol Twice weekly dosing for full course of treatment should not occur unless done in context of formal research Treatment Recommendations (2) New Patient Groups Presumed or known drugsusceptible and living with HIV or in a high HIV prevalence setting TB treatment regimens Initial phase (2 mos.) Continuation phase Optimal HRZEa Optimal HR, 4 mos. Acceptable HRZEa Acceptable HRc 7 mos. Treatment Recommendations (3) TB treatment regimens New Patient Groups Setting with high levels of INH resistance where DST at start of treatment is not universal Settings where >3% of new patients have MDR Initial phase (2 mos.) Continuation phase Acceptable HRZEa daily Acceptable HRE, 4 mos. Obtain DST at the start if therapy DST = drug susceptibility testing Standard 8: Drug Formulations and Doses The doses of anti-tuberculosis drugs used should conform to international recommendations Fixed-dose combinations (FDC) of two (INH and RIF), three (INH, RIF, and PZA), and four (INH, RIF, PZA, and EMB) drugs are highly recommended Adult Daily Dose of FDC Tabs Body Weight KG Initial Phase [RHZE] Continuation Phase [RH] 30-37 2 2 38-54 3 3 55-74 4 4 ≥75 5 5 12-15 Pills Per Day to Only 4-5 FDCs Image source: Pierre Virot Image source: Jad Davenport Dose Recommendations Doses of First-line Anti-TB Drugs in Adults Drugs Recommended dose in mg/kg body weight Daily (mg/kg range) 3 x weekly Isoniazid (INH, H) 5 (4-6), max 300mg/day 10 (8-12) max 900/dose Rifampicin (RIF, R) 10 (8-12), max 600mg/day 10 (8-12) max 600/dose Pyrazinamide (PZA, Z) 25 (20-30), max 2000mg/day 35 (30-40) max 3000/dose Ethambutol (EMB, E) 15 (15-20), max 1600mg/day 30 (25-35) max 2400/dose Streptomycind (SM, S) 15 (12-18), max 1000mg/day 15 (12-18) Treatment Outcomes for Pulmonary TB 1.2% 10% 50% Dead 64% 98% Sputum positive 32% 18% Sputum negative 20% 0.8% No Poor Good Chemotherapy Chemotherapy Chemotherapy Grzybowski S et al, Bull Int Union Tuberc 1978; (53)2: 70-5 ISTC Standard 10 Response to therapy in patients with pulmonary tuberculosis should be monitored by follow-up sputum microscopy (two specimens) at the time of completion of the initial phase of treatment (two months) If the sputum smear is positive at completion of the initial phase, sputum smears should be examined again at 3 months and, if positive, culture and drug susceptibility testing should be performed ISTC Standard 10 (2) In patients with extrapulmonary tuberculosis and in children, the response to treatment is best assessed clinically. Required Monitoring New sputum smear-positive patients should have sputum smear exams monthly Other clinical markers of treatment response to monitor: • Weight at initiation of treatment, then monthly until weight is stable • Review of TB symptoms present at time of diagnosis and documentation when symptoms resolve With the RIF-based regimen throughout, WHO no longer recommends extending initial phase if smear-positive at 2 months Treatment of Extrapulmonary TB (EPTB) and Special Situations Treatment: Extrapulmonary TB In general, EPTB is treated the same as PTB except: • Streptomycin replaces EMB when treating CNS TB • Corticosteroids may be useful in some forms of EPTB • Some experts recommend extending the duration of therapy in patients with: – CNS tuberculosis – Bone/joint tuberculosis WHO no longer recommends option to omit EMB during initial phase in HIV-negative EPTB patients Treatment: Extrapulmonary TB (2) Treatment Duration and Use of Steroids Site Length of Rx (mos.) Corticosteroids 6 No 6-9 No Pleural 6 No Pericarditis 6 Yes 9-12 Yes Disseminated 6 No Genitourinary 6 No Abd/Peritoneal 6 No Lymph node Bone/Joint CNS ATS/CDC/IDSA 2003 New Sputum Smear-Negative PTB If also HIV-positive and/or severely ill: • Obtain CXR and sputum TB culture • Start antibiotic treatment for pneumonia (nonFQN) and if CXR findings suggest that TB is likely (positive or negative TB smear) then register as a new TB case and initiate TB treatment If HIV-negative and/or mild/moderate illness: • Obtain CXR and sputum TB culture • If CXR findings suggest that TB is likely or TB culture is positive then register as a new case and initiate TB treatment Treatment During Pregnancy Risk to mother and fetus is far greater from untreated TB than from the drugs used to treat TB: Increased risk of spontaneous abortion Increase in perinatal mortality Small for gestational age births Increased maternal morbidity Congenital TB Increased risk of perinatal and early postnatal transmission Treatment During Pregnancy (2) Isoniazid (INH), rifampicin (RIF) and ethambutol (EMB) are known to be safe for administration during pregnancy Supplement with pyridoxine 25mg/day Streptomycin should be avoided Monitor for signs of hepatotoxicity during pregnancy and immediate post-partum Breastfeeding Most of the TB medications are secreted into breast milk but not in significant concentrations (usually <1-12% of levels measured in the serum) Levels are not likely to lead to toxicity in the infant Levels will not be sufficient to protect the infant – infant should receive IPT Supplement Mom with B6 while breastfeeding TB Treatment and Liver Disease Use standard short-course regimen for patients without clinical evidence of chronic liver disease but history of: • Hepatitis virus carriage • Past history of acute hepatitis • Current excessive alcohol consumption Hepatotoxic reactions are more common in these patients and should therefore be anticipated TB Treatment and Liver Disease Use a liver sparing regimen for patients with established chronic liver disease: • Two hepatotoxic drugs – 9HR+E (until or unless INH susceptibility documented) – 2HRSE/6HR – 6-9RZE • One hepatotoxic drug – 2HES/10HE • No hepatotoxic drugs – 18-24SE+Fluoroquinolone Hepatitis Hepatitis (asymptomatic elevation AST/ALT occurs in 20% patients on 4 drugs) • Drug induced hepatitis = AST or ALT ≥3 times upper limits of normal in the presence of symptoms OR >5 times if asymptomatic • INH, PZA and RIF can all cause hepatotoxicity – Hepatitis from INH is age related, from PZA is dose related, and RIF is unpredictable and less common TB Treatment and Hepatitis If >3x normal with symptoms or > 5x normal without symptoms: • stop all anti-TB medications and evaluate patient • refer patient to doctor for clinical evaluation • try to rule out other causes of acute liver disease • if severely ill, may start 3 non-hepatotoxic drugs • after AST < 2 times upper limit of normal — re-challenge drugs one-by-one starting with drugs that are not hepatotoxic The Renal Impaired TB Patient Patients with end stage renal disease (ESRD) have increased risk for progression to active TB disease • Risk is 10 – 25 times greater for persons with ESRD than in the general population The Renal Impaired TB Patient (2) If CrCl <30ml/min., including hemodialysis patients, administer anti-TB treatment thrice weekly after dialysis at the following doses: • Isoniazid and rifampicin 10mg/kg • PZA 25-mg/kg • EMB 15-mg/kg Include supplemental pyridoxine 25-50mg with the thrice weekly regimen to prevent peripheral neuropathy Summary Appropriate treatment and assessment of adherence to treatment is an important public health issue The use of internationally accepted first-line treatment regimens is associated with a high cure rate and a low risk of acquired drug resistance Pulmonary and EPTB are generally treated with the same regimens (Exception: extended duration in meningeal/CNS and bone/joint disease) Monitoring for both response to treatment and for potential adverse events is essential Summary: ISTC Standards Covered* Standard 7: Practitioners assume an important public health responsibility in ensuring both appropriate treatment regimens and assessment of treatment adherence for their patients. Standard 8: All patients who have not been previously treated should receive an internationally accepted treatment regimen: Initial phase: 2 months INH, RIF, PZA, and EMB Continuation phase: 4 months INH and RIF * Abbreviated versions Summary: ISTC Standards Covered* Standard 8: (continued) The doses of anti-tuberculosis drugs used should conform to international recommendations. Fixed-dose combinations are highly recommended. Standard 10: Response to therapy in patients with pulmonary TB should be monitored by follow-up sputum microscopy (2 specimens), if positive, sputum smears should be examined again at 3 months. If positive, culture and DST should be performed. * Abbreviated versions