* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download slides
Survey
Document related concepts
Transcript
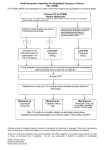
Prasugrel - New Pharmacodynamic Data: SWAP and OPTIMUS-3 Trials, and Ticagrelor on Platelet Function Dominick J. Angiolillo, MD, PhD, FACC, FESC, FSCAI Director of Cardiovascular Research Assistant Professor of Medicine University of Florida College of Medicine-Jacksonville DISCLOSURES Dominick J. Angiolillo, MD, PhD Within the past 12 months, the presenter or their spouse/partner have had a financial interest/arrangement or affiliation with the organization listed below. Honoraria/Lectures: Bristol Myers Squibb, Sanofi-Aventis, Eli Lilly Co, Daiichi Sankyo, Inc., Advisory Board: Bristol Myers Squibb, Sanofi-Aventis, Eli Lilly Co, Daiichi Sankyo, Inc., The Medicines Company, Portola Pharmaceuticals, Novartis, Arena Pharmaceuticals, Accumetrics, Medicure Research Grants: GlaxoSmithKline, Otsuka, Accumetrics, Eli Lilly Co, Daiichi Sankyo, Inc., The Medicines Company, Portola Pharmaceuticals, Schering-Plough, Astra Zeneca, Johnson & Johnson I intend to reference unlabeled/ unapproved uses of drugs or devices in my presentation. I intend to reference Ticagrelor, Elinogrel, Cangrelor, and TRA. The SWitching Anti Platelet (SWAP) Study Angiolillo DJ et al. Presented at American Heart Association Nov 2009 Angiolillo DJ et al. J Am Coll Cardiol 2010 (in press) SWAP Background • Prasugrel is associated with greater P2Y12 receptor blockade, greater platelet inhibition, and reduction of ischemic complications compared to clopidogrel in ACS patients undergoing PCI. • Pharmacodynamic studies have shown that treatment with prasugrel results in higher and more consistent levels of platelet inhibition than clopidogrel, even when higher than approved loading or maintenance clopidogrel doses are used. • The effects of switching ACS patients during maintenance phase clopidogrel therapy to prasugrel on platelet function are unknown. SWAP Objectives • PRIMARY – Evaluate the pharmacodynamic consequence of switching patients on clopidogrel 75 mg maintenance dose (MD) following an ACS event to prasugrel 10 mg MD therapy with or without a loading dose (LD). • SECONDARY – Evaluate the effect of switching from clopidogrel to prasugrel on thienopyridine pharmacodynamic poor responder rates. SWAP Study Design Patient eligible for enrollment 30-330 days post ACS (Must be prescribed clopidogrel 75 mg) Daily aspirin 81-325 mg to continue throughout study Clopidogrel 75 mg MD x 13-15 days Baseline platelet function studies at end of clopidogrel run-in (139 patients randomized) Clopidogrel 75 mg MD x 13-15 days N=33 Prasugrel 10 mg MD x 13-15 days N=36 Prasugrel 60 mg LD, 10 mg MD x 13-15 days N=31 Platelet function studies at 2 hours, 24 hours, 7 and 14 days SWAP Maximum Platelet Aggregation (%) Maximum Platelet Aggregation (20 µM ADP) 100 Placebo LD/Clopidogrel 75 mg MD (n=33) Placebo LD/Prasugrel 10 mg MD (n=36) Prasugrel 60 mg LD/10 mg MD (n=31) 80 60 * * 40 *† *† ** 20 * p<0.0001 vs clopidogrel 75 mg MD † p<0.0001 vs prasugrel 10 mg MD Mean±SE 0 02 12 Time, hours 24 4 6 8 10 12 14 Time, days Similar findings obtained with MPA to 5 µM ADP, VASP PRI, and Verify Now® PRU SWAP Thienopyridine Poor Responders Poor Responder Rate at Baseline and 7 Days by Multiple Platelet Function Assays. 100 Poor Responder Rate (%) 80 60 40 20 * † † 0 Baseline 7 days Baseline 7 days Baseline 7 days Baseline 7 days MPA >65% MPA >50% PRU >235 PRI >=50% Clopidogrel 75 mg Combined Prasugrel Groups Poor responders were defined at baseline (prior to randomization) and at 7 days following start of study drug. *p<0.05; †p≤0.001; MPA= Maximal platelet aggregation to 20 mM ADP by LTA; PRU=P2Y12 reaction units; PRI=platelet reactivity index SWAP Conclusions • Switching ACS patients during maintenance phase clopidogrel therapy to prasugrel: resulted in significantly lower platelet aggregation by one week, regardless of the platelet function test used. achieved more rapid platelet inhibition within 2 hours using a LD of prasugrel than without a LD. associated with reduced poor responder rates. Comparison of the Effects of Prasugrel with Clopidogrel on Platelet Function in Coronary Artery Disease Patients with Type 2 Diabetes Mellitus — Third Optimizing Anti-Platelet Therapy In Diabetes MellitUS (OPTIMUS-3) Angiolillo DJ et al. Presented at American Heart Association Nov 2009 OPTIMUS-3 Background • Patients with diabetes are characterized by enhanced platelet reactivity and decreased response to clopidogrel compared to patients without diabetes. • • In TRITON-TIMI-38, the subset of patients with diabetes showed a 30% reduction in the primary composite endpoint of cardiovascular death, MI, or stroke with prasugrel compared with clopidogrel • Specifically designed, randomized studies of pharmacodynamic effects of prasugrel in patients with diabetes and coronary artery disease (CAD) have not been previously performed. OPTIMUS-3 Primary Objective • Compare the pharmacodynamic effects of a 60-mg loading dose (LD) of prasugrel vs. clopidogrel 600 mg LD in subjects with diabetes mellitus and CAD using inhibition of platelet aggregation (IPA), as measured by the VerifyNow® P2Y12 assay at 4 hours post-LD. OPTIMUS-3 Study Design Randomized, two-way, crossover, double-blind study in patients with diabetes and CAD Enrollment (n=35) Randomization Prasugrel 60 mg LD/10 mg MD Clopidogrel 600 mg LD/150 mg MD Platelet function studies drawn at baseline, 1 hr post-LD, 4 hrs post-LD, 24 hrs post-LD, 1 week post-LD Platelet function studies drawn 1 week post-drug discontinuation Platelet function studies drawn 1 week post-drug discontinuation 2 Week Washout Prasugrel 60 mg LD/10 mg MD Clopidogrel 600 mg LD/150 mg MD Repeat platelet function studies as above Including 1 week post-discontinuation MD= maintenance dose OPTIMUS-3 Verify Now®-P2Y12 % Inhibition 350 VN-P2Y12 % Inhibition PRU 100 300 250 80 200 60 150 B A LD LD *** MD MD Washout Washout *** *** *** *** *** 40 100 20 50 0 0 *** *** Mean±SE 0 4 24 0 4 Hours post LD 24 Hours post LD ***p<0.0001 7 days 7 days No study drug No(7 study drug days) (7 days) prasugrel 60 mg LD/10 mg MD clopidogrel 600 mg LD/150 mg MD Similar findings obtained with MPA to 5 and 20 µM ADP, VASP PRI, and Verify Now® PRU OPTIMUS-3 Conclusions • In patients with diabetes mellitus and CAD, standard dose prasugrel is associated with significantly greater inhibition of platelet function than double-dose regimens of clopidogrel in the acute and maintenance phases of treatment. New pharmacodynamic studies on Ticagrelor PLATO PLATELET ONSET/OFFSET RESPOND Presented at American Heart Association Orlando, Fl - Nov 2009 PLATELET REACTION UNITS (PRU) PLATO PLATELET – VerifyNow P2Y12 assay Maintenance therapy with clopidogrel (C) vs ticagrelor (T) 500 **** **** 400 300 235 PRU 200 100 0 C T Trough Storey RF, Angiolillo DJ, et al. Presented at American Heart Association Nov 2009 C T Peak ONSET/OFFSET Study 100 Loading Dose Last Maintenance Dose * * * * 90 * Ticagrelor (n=54) * Clopidogrel (n=50) Placebo (n=12) * 80 † 20 µM ADP- Final Extent * 70 IPA % 60 50 * 40 ‡ 30 † 20 10 0 0 .5 1 2 Onset Gurbel PA et al. Circulation 2010 4 8 24 6 weeks 0 2 4 8 Maintenance 24 48 72 120 168 240 Offset Time (hours) RESPOND • Patients with stable CAD on aspirin (n=98) were administered a 300 mg Clopiogrel load and Non-responders (NR) identified (10% absolute change from baseline in MPA by LTA (20 µM ADP) at 6 – 8 h). • Two-way crossover design (Phase I and Phase II): NR (n=41) and responders (R) (n=57) were randomized to receive either Clopidogrel 600 mg load/75 mg qd for 14 days or Ticagrelor 180 mg load/90 mg bid for 14 days. • Ticagrelor was associated with significantly greater platelet inhibition in both clopidogrel responders and nonresponders (effect not influenced by clopidogrel response status). • Clopidogrel nonresponse was uniformly overcome by ticagrelor. • During switching of therapies, ticagrelor produced a rapid enhancement in platelet inhibition in both clopidogrel responders and nonresponders. Gurbel PA et al. Circulation 2010 (in press)