* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download L4 - Carboxylic Acids
Survey
Document related concepts
Transcript
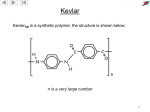
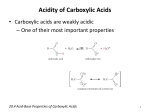
Carboxylic Acids Carboxylic acids and Their Salts • Have hydroxyl group bonded to carbonyl group. • Tart tasting. • Carboxylic acids are weak acids.. Naming Carboxylic Acids Find the longest carbon chain that contains the –COOH group. Drop the –e from the end of the alkane name and substitute –oic acid. Number the longest chain. Carbon number 1 is the carboxyl carbon. Name and number other substituents. Examples: Aromatic acid names are derived from the parent compound, benzoic acid. Example: 1 Name the Following: Physical Properties of Carboxylic Acids 1. OH OH 2. :O: 3. OH 4. HO The carboxylate group is very polar. Hydrogen bonding between carboxylates creates dimers (two identical molecules bonded together). : 5. :Br: HO OH :O: :O: Notice, The boiling points of carboxylic acids are higher that that of alcohols This gives carboxylic acids high boiling points (greater than alcohols). Physical Properties, cont. Carboxylate groups can hydrogen bond with water. If the hydrophobic –R group is not too large, carboxylic acids are very water soluble. Long chained carboxylic acids were first extracted from fats and are called “fatty acids” Insoluble carboxylic acids, or fats, form a monomolecular layer when placed in water due to the polar carboxyl group. Acidity of Carboxylic Acids Carboxylic acids behave as weak acids (low dissociation), forming carboxylate ions in water. 2 Examples: Carboxylic Acid Reactions Formation Formation of a carboxylate salt (Ionization substitution) Carboxylic acids react readily with strong bases (NaOH, KOH) to form salts. Acid dissociation results in the formation of a carboxylate ion Examples: Carboxylate Salts • To name a carboxylic acid salt, name the metal ion first and change the –ic ending of the acid to –ate. Example: Useful Carboxylic Acid Salts Na and K salts of long-chain acids are used as soaps (sodium stearate). Calcium and sodium propanoate are used as preservatives in bakery products. Sodium benzoate is a food preservative used in ketchup and soda pop. Zinc 10-undecylenate is used to treat athelete’s foot. overheads Hey kids, ask me about micelles and how soaps get us so squeaky clean! 3