* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download AP Chapter 9 Molecular Shapes
Survey
Document related concepts
Transcript
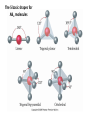
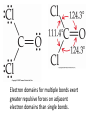
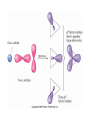
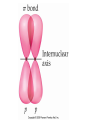
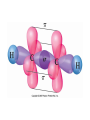
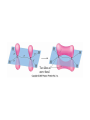
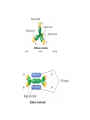
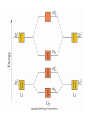
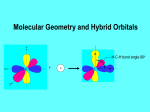
AP Chapter 9 Molecular Geometry and Bonding Theories Molecular Shapes • The 3-dimensional shapes and sizes of molecules are determined by their bond angles and bond lengths. • Molecules with a central atom surrounded by n atoms B, denoted ABn, adopt many different geometric shapes, depending on the value of n, and on the atoms involved. • The majority have one of 5 basic shapes: linear, trigonal pyramidal, tetrahedral, trigonal bipyramidal and octahedryl. The 5 basic shapes for ABn molecules Additional molecules can be made by removing corner atoms from the 5 basic shapes. This one starts with a tetrahedral. The VSEPR Model • VSEPR – valence shell electron-pair repulsion model. • Molecular geometries are based on the repulsions between electron domains. (Like charges repel.) • Shared pairs (bonded pairs) and unshared pairs (nonbonding pairs) create electron domains around the central atom. VSEPR • Electron domains remain as far apart as possible. • Nonbonding pairs of electrons (unshared pairs) exert more repulsions than bonding pairs (shared pairs.) • Electron domains from multiple bonds exert slightly more repulsions than those from single bonds. VSEPR • The arrangement of electrons around a central atom is called electron-domain geometry. • The arrangement of atoms is called the molecular geometry. In general, each non-bonding pair, single bond or multiple bond produces an electron domain around the central atom. Balloons tied together at their ends naturally adopt their lowest-energy arrangement. Electron Domains • The best arrangement of a given number of electron domains is one that minimizes the repulsions among them. This is why the balloon analogy – they have similar preferred geometries. Predicting Shapes 1. Draw the Lewis structure of the molecule or ion and count the total number of electron domains around the central atom. 2. Determine the electron-domain geometry by arranging the electron domains around the central atom so repulsions are minimized. (Table 9.1) 3. Use the arrangement of the bonded atoms to determine the molecular geometry. The Effect of Nonbonding Electrons and Multiple Bonds on Bond Angles The bond angles decrease as the number of nonbonding electron pairs increase. Nonbonding pairs of electrons • Nonbonding pairs of electrons take up more space than bonding pairs. • The electron domains for nonbonding electron pairs exert greater repulsive forces on adjacent electron domains and tend to compress the bond angles. Electron domains for multiple bonds exert greater repulsive forces on adjacent electron domains than single bonds. Molecular Shape and Molecular Polarity Covalent Bonding and Orbital Overlap • Valence bond theory states that covalent bonds are formed when atomic orbitals on neighboring atoms overlap one another. Hybrid Orbitals • This involves mixing s, p and sometimes d orbitals. • Linear = sp • Trigonal planar = sp2 • Tetrahedral = sp3 • Trigonal bipyramidal = sp3d • Octahedral = sp3d2 Multiple Bonds • Sigma bonds (σ) – the overlap of 2 s orbitals • Pi bonds (π) – the sideways overlap of p orbitals. π bonds in Benzene rings and resonance structures Resonance structures Hybridization Involving Triple Bonds Sigma and Pi Bonds • Double bonds consist of one δ bond and one π bond • A triple bond consists of one δ bond and two π bonds • π bonds must lie in the same plane, therefore, the presence of π bonds makes the molecule slightly rigid. Molecular Orbitals • In the Molecular Orbital theory, electrons exist in allowed energy states called molecular orbitals (MO). • They can be spread among all the atoms in a molecule, they have a specific amount of energy and can hold a maximum number of 2 electrons. Molecular Orbitals • The combination of two atomic orbitals leads to 2 MOs, one with low energy and one with higher energy. • The lower energy MO concentrates the charge density in the region between the nuclei and is called a bonding molecular orbital. • The higher energy MO excludes electrons from the region between the nuclei and is called antibonding molecular orbitals. Bond orders • When the appropriate number or electrons are placed into the MOs, bond orders can be calculated, which is half the distance between the number of electrons in bonding MOs and the number of electrons in the antibonding MOs. • A bond order of 1 corresponds to a single bond and so on. They can be fractions! Paramagnetism and Diamagnetism • Paramagnetism – the attraction of a molecule by a magnetic field due to unpaired electrons. • Diamagnetism – in molecules where all the electrons are paired, the molecules exhibit a weak repulsion from a magnetic field.