* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Amyloidosis - chem.uwec.edu
Onchocerciasis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
Visceral leishmaniasis wikipedia , lookup
Creutzfeldt–Jakob disease wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
Bovine spongiform encephalopathy wikipedia , lookup
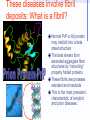
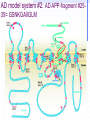
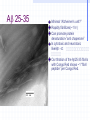
Amphotericin B Two-edged sword or Swiss army knife? Bridging the gap between Alzheimer’s and Prion diseases. What are some Amyloid Diseases? Alzheimer’s disease Atrial Amyloidosis Hereditary Renal Amyloidosis Secondary Systematic Amyloidosis Injection-Localized Amyloidosis What are some Prion Diseases? Chronic Wasting Disease (CWD) Scrapie BSE- Mad Cow Disease Kuru Creutzfeldt-Jakob Disease Amyloids vs Prions Prions are known to be infectious in their spreading to different hosts, e.g. CWD, BSE, kuru, etc… Amyloid diseases not thought to be infectious agents but…. Amyloids vs. Prions-news! It has been recently shown that mice who were fed amyloid fibrils (from the spleen of infected mice) orally (in H2O) developed amyloid deposits upon stimulation. Amyloids vs. Prions, pt. II Similar propagation of fibrils in vitro. Similar symptoms (inflammatory neuropathies) and adverse affects from having the disease. These diseases involve fibril deposits: What is a fibril? Normal PrP or Ab protein may misfold into a beta sheet structure The beta sheets form extended aggregate fiber structures by “recruiting” properly folded proteins These fibrils are protease resistant and insoluble This is the most prevalent characteristic of amyloid and prion diseases. Alzheimer’s Disease Amyloid hypothesis Possible approaches to treating Amyloid and Prion Diseases. 1. Physical blockage of fibril growth (by small molecules). 2. Alter processing/clearance of protein (enzyme inhibitors) 3. Damage Control of existing fibrils(?) (NSAIDs -- Non-steroidal AntiInflammatory Drugs, Statins) 4.Symptomatic (anticholinergics) 5. Effecting an immune response. Specific-vaccine Non-Specific-general stimulant How would Physical Blockage work? Congo Red: a molecule that binds to and inhibits fibril growth. Congo Red is an azo dye that was used by Alois Alzheimer to characterize brains with Alzheimer’s disease Some other in vitro success using N-methylated peptides, anti-sense peptides and other small molecules. AD model system #1: Characterizing fibrils Congo Red binds to fibrils very specifically. Absorbance at 540 nm can be used to quantitate [fibril] formation The Insulin Fibril system was used as it is a proven amyloid model system. Hypothesis: AmB is one of the only agents known to slow prion diseases in animal models (hamster,mouse)! Perhaps AmB could act by the 1st mechanism by binding to existing fibrils and preventing growth, thus preventing the propagation of the disease. Why AmB? Amphotericin B is an antifungal drug used to treat immunocompromised people (AIDS, cancer) who have deep fungal infections. Amphotericin B is also an interesting molecule that has a polyene side and a polyol side, which may hint to its binding characteristics. It is also a relatively inexpensive drug that is already on the market. Does AmB bind to fibrils? 417 nm Yes it does! Although the actual binding site(s) are unknown, it does bind to fibrils specifically and not to protein in native form. 1 mole AmB/2 mole insulin Kd~1.1µM Does AmB B inhibit insulin Fibril Formation? No it doesn’t. Luckily it does not induce fibrils either Thus, AmB neither inhibits nor promotes fibril formation under these conditions (pH 2-HCl). AD model system #2: AD APP-fragment #2535= GSNKGAIIGLM Ab 25-35 Minimal “Alzheimer’s unit?” Rapidly fibrillizes(~1 hr) Can promote protein denaturation-”anti chaperone” Is cytotoxic and neurotoxic likeAb Our titration of the Ab25-35 fibrils with Congo Red shows ~1 “fibril peptide” per Congo Red. Ab 25-35 fibrils do bind AmB But…does AmB B inhibit Ab25-35 Fibril Formation? But…does AmB B inhibit Ab25-35 Fibril Formation? Yes it does!! At reasonable therapeutic concentrations, too (7.5 and 15µM) However, it only delays the onset of fibrillogenesis;it does not change final concentration Ab 1-40 and PrP peptides will be the real test. How else might Amphotericin B work in addition to fibril inhibition? Amphotericin B may work non-specifically. Because of its properties and known prior use, it may be able to activate the generalized immune system acute phase response (IL-1, IL-6) and promote removal of amyloid or prion deposits. Fibrils may also help sequester AmB in tissues most strongly affected. Previous reports have shown that introducing AmB 2 weeks prior or immediately with the systematic prion inoculum effectively slows the course of the prion infection. Later treatment is less effective. It might bind to and modify oligomer ion channel properties associated with amyloid diseases. AmB would selectively associate with cholesterol-rich membrane “rafts” and alter processing (Ab) or conversion(PrP). Vaccination analog, i.e. could AmB-protein adduct resemble a fibril and lead to antibody/T-cell response?(But.. SCID mice.) Conclusions Amphotericin B binds specifically to two different b fibril models suggesting a possible mechanism for its demonstrated antiprion activity. This is further supported by the kinetic inhibition of Ab 25-35 fibrillization by AmB and suggests a possible screening assay for future potential drugs Further testing on PrP and Ab 2 confirm this hypothesis As a sidelight, it seems worth investigating whether the fungal amyloid-like protein hydrophobin may be important to AmB susceptibility/resistance THANKS ! Projects in our lab Physical characterization of Amphotericin B drug delivery vehicles (stability, ion channel formation) Pharmaceutical testing of new AmB preparations (with K. Wasan) Cytokine expression profiles caused by AmB preps in immune cells (with L. Turtinen) Localization and metabolism of liposomal doxorubicin in single cells (with E. Arriaga) AmB potential effects on amyloid diseases