* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 20110610_PDF design - international journal of advances in
Survey
Document related concepts
Pharmacometabolomics wikipedia , lookup
MTOR inhibitors wikipedia , lookup
Biochemistry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Peptide synthesis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Proteolysis wikipedia , lookup
Drug design wikipedia , lookup
Drug discovery wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Transcript
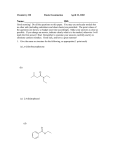
DESIGNING OF NOVEL PEPTIDE DEFORMYLASE INHIBITORS COMPARATIVE MOLECULAR FIELD ANALYSIS Itishree Shantanu Vaidya1*, Kaumudi Sumedh Puranik2, Krishnacharya Akamanchi2 1 Dr. L.H. Hiranandani College of Pharmacy, CHM Campus, Ulhasnagar, District: Thane, Maharashtra PIN: 421003 2 Pharmaceutical Division, Institute of Chemical Technology, Matunga, Mumbai-400019 Address for correspondence: *Dr. (Mrs.) Itishree Vaidya, Dr. L.H. Hiranandani College of Pharmacy, CHM Campus, Ulhasnagar, District: Thane, Maharashtra PIN: 421003 Tel: 0251-2561463/ 9819287323 Tel Fax: 0251-2561341 Email: [email protected] BY A ABSTRACT Peptide deformylase (PDF) represents the most promising bacterial target in the search of novel antibiotics lacking cross resistance to existing drugs. PDF inhibitors reported till dates are peptide and non-peptide inhibitors. To figure out the structural requirements of PDF inhibitors belonging to non-peptidic class and to optimize their Escherichia coli peptide deformylase inhibitory activity comparative molecular field analysis(CoMFA) on twenty low molecular weight -Sulfonyl and sulfinylhydroxamic acid derivatives was performed. A CoMFA model showed considerable internal as well as external predictive ability (r2cv = 0.707, r2pred = 0.826). CoMFA study helped in designing novel PDF inhibitors and their PDF inhibitory activities are also predicted. KEYWORDS Peptide deformylase, non-peptide inhibitors, hydroxamic acid, antibacterial, comparative molecular field analysis INTRODUCTION The emergence of antibiotic-resistant bacteria has created an urgent demand for new antibacterial agents with novel mechanisms of action. Bacterial genomics has revealed a plethora of previously unknown targets of potential use in the discovery of novel antibacterial design. The vital features for the identification of good target agreed are i) present in most human pathogens (wide –spectrum effects), ii) absent from human cells, iii) part of an essential pathway in the pathogens, iv) not be inhibited by widely used antibiotics, v) easy to assay invitro and invivo, vi) highly specific for the pathogen and non-toxic to humans and vi) not result in resistance or bypass processes if inactivated. Peptide deformylase (PDF; EC 3.5.1.31) fulfils the criteria listed above and is likely the most attractive bacterial target to deliver the next class of novel antibacterial drugs. 1-4 The protein synthesis processes for bacterial and mammalian cells are very similar. Both utilize the same amino acids and codons and share the same mechanism for elongation. However, a major difference between bacterial protein synthesis and mammalian cytosol protein synthesis is the use of formylmethionine as the initiator. Unlike cytosol protein synthesis in mammalian cells, which is initiated with methionine, protein synthesis in bacteria is initiated with N-formylmethionine which is generated through enzymatic transformylation of methionyl-tRNA by formylmethionine tRNA transferase. PDF is a bacterial metalloenzyme and is responsible for incorporating or removing Formyl group into bacterial methioninepolypeptide chain5. The N-formyl methionine of the nascent protein in bacteria is removed by the sequential action of PDF and a methionine amino peptidase in order to afford the mature protein. This formylation-deformylation cycle is essential for bacterial growth and is conserved among all studied bacterial species. Previous reports indicate that this cycle is not required for mammalian cells. The specific bacterial requirement for PDF in protein synthesis provides a rational basis for selectivity, making it an attractive drug discovery target. PDF is essential for bacterial survival; either deletion of def gene or treatment with a PDF inhibitor prevents bacterial growth.6- 8 Rational and design strategy: Using mechanistic information about the reaction catalyzed by PDF, together with an understanding of the general principles of metalloprotease inhibition, others have constructed several chelatorbased inhibitor libraries with chelating pharmacophore element that can bind to the metal ion at the active centre of PDF, the N-alkyl group mimics the methionine side chain, and domains of the inhibitor that can provide additional binding energy, selectivity, and favourable pharmacokinetic properties9-12. With the available 3D-QSAR facilities in house we have decided to explore structural requirements of the PDF inhibitors. In the present investigation our interest was to develop a Comparitive Molecular Field Analysis (3D-CoMFA) for reported PDF inhibitors, which would give us better insight for designing of newer PDF inhibitors. To fulfil this objective, Tripos’s SYBYL 6.6 program was used to develop 3D-QSAR model. Dataset: A set of 20 compounds belonging to -Sulfonyl and sulfinylhydroxamic acid derivatives were selected for study from literature13. Table I gives biological activities of these compounds. The biological activity expressed as log BA, where BA is IC50 for PDF of Escherichia coli and used as a dependent variable. pIC50 = -log10 IC50 where IC50 is millimolar concentration of the inhibitor producing 50% inhibition Method: All computational studies were performed on Silicon Graphics INDY R5000 workstation using SYBYL6.6 molecular modelling software from Tripos Inc., St. Louis, MO. 14 The 3D structures of molecules were built from fragments in SYBYL database. Each structure was fully geometry optimized using standard Tripos force field with distance dependent dielectric function and distance dependent dielectric function and 0.001Kcal/mol energy gradient convergence criterion. Partial atomic charges were computed by semiempirical molecular orbital method using MOPAC 6.0 program. The charges were computed using the PM3 model Hamiltonian. The minimized structures were subjected to systematic search routine of all rotatable bonds in 10, increment from 0 to 360. Conformational energies were computed with electrostatic term, the lowest energy structure finally minimized was used in superimposition. The most active compound 12 was selected for this purpose. Development of predictive 3D-QSAR predictive model is essentially alignment sensitive that defines the putative pharmacophore of the series of ligands under investigation. Each analogue was aligned to the template molecule 12 by Atom fit method in SYBYL. CoMFA analysis: CoMFA steric and electrostatic field energies were calculated using the sp3 carbon probe atom using a Vander Wall’s radius of 1.52 A. The energies were truncated to ±30Kcal/mol and the electrostatic contributions were ignored at the lattice intersection with maximal steric interaction. The CoMFA fields generated were automatically scaled by CoMFA-STD method in SYBYL. Partial least squares analysis (PLS): The CoMFA descriptors served as independent variable(X )and pIC50 as dependent variable(Y) in PLS regression analysis for deducing 3-D QSAR models. PLS analyses were performed following CoMFA standard implementation in SYBYL. Normally cross validation is used to check the predictivity of the derived model. The results of the analysis correspond to the regression equation with thousands of coefficients. The predictive values of the model were evaluated using Leave-one-out (LOO) cross validation method. The number of components leading to the highest cross validated r2 and lowest standard error of prediction (SEP) was set as optimum number of components (Nc) in PLS analysis. For all conventional analyses (no cross-validation) the minimum sigma stanadard deviation threshold was set to 2.0 Kcal/mol. The cross validated r2 is defined as r2cv= 1-PRESS / Σ(Y- Ymean)2 where PRESS= Σ(Y- Ypred)2 Ymean: Mean biological activity, and Ypred: Predicted biological activity Predictive ability of CoMFA: It is determined from test set molecules that were not included in training set. These molecules were aligned and their activities were predicted by PLS analysis. The predictive r2 (r2pred) is defined as: r2pred = (SSD-PRESS)/SSD Where, SSD: sum of squared deviations between the biological activity of test set and mean activity of training set molecules PRESS: Sum of squared deviation between actual and predicted activities of test set molecules. Figure 1 gives predicted activity for the compounds belonging to training set and Figure 2 gives the predicted activity for test set molecules. Test set comprised of compounds namely 10, 14, 15 and 20 and rest as training set. CoMFA Contour maps: In order to visualize the derived 3D QSAR model, CoMFA steric and electrostatic fields from PLS analysis, contour maps of the product of standard deviation associated with the CoMFA column and coefficient at each lattice point were generated. The contour maps are plotted as percentage contribution to the QSAR equation and are associated with the difference in the biological activity. The contour maps generated gives important ideas about the effect of substituent on various positions in the compound. RESULTS AND DISCUSSION The model generated by CoMFA is summarized in table 2. The model was analyzed for the predictive ability for the training set as well as test set molecules. Final model was selected primarily based on the values of better cross-validated r2 and predictive r2 (all of these values are highlighted in table 2.) In this CoMFA analysis both steric and electrostatic fields contribute to QSAR equation by 63.9% and 36.1% respectively, suggesting variation in the biological activity of compounds is dominated by differences in steric interactions. Figure 3, 4 and 5 give contour maps for steric, electrostatic fields for most active (compound 12) and steric fields for least active (compound 6) compound in the series respectively. Bulky substituent in the region shaded yellow is likely to decrease biological activity. It is observed from figure 5 that Compound 6 containing R1 substituent as hexyl is least active because its hexyl chain is placed in sterically unfavourable region. Also the activity of these inhibitors is strongly influenced by nature of substituent R2. Green contours are exactly located where R2 substituent extends in the space clear from figure 3. Really substituent R2 occupies the same position in the enzyme as methionine side chain of the natural substrate. The most active member of the series compound 12 with R1 as 4-AcNH-phenyl mimic the peptide backbone of the substrate and projects in sterically favoured region as seen from figure 3. The sulfone oxygen of the inhibitor 12 is oriented exactly in the high red contour density suggesting it is involved in bonding interaction with enzyme. The results of the model are found to be predictive and can be used to design novel PDF inhibitors. Based on literature on PDF inhibitors and our current CoMFA studies for PDF inhibitors and we have designed molecules and three of those designed NCEs are shown in figure 6. A current CoMFA study is used to predict their activities are given in table III. Out of the three compounds designed compound III is showing highest PDF inhibitory activity. Steric bulk for III and II are comparable and more than compound I. But compound III is electronically also in favourable region because of position of hydroxyl group. CONCLUSION: CoMFA analysis of twenty -sulfonyl and sulfinylhydroxamic acid derivatives produced good model with high predictive abilities (r2cv = 0.707, r2pred = 0.826). The contour diagrams obtained from CoMFA field contribution are mapped back onto structural features accounting for activity trends among the inhibitors. On the basis of spatial arrangement of field contribution novel molecules are designed and predicted for PDF inhibitory activity. The information is very crucial in the design and development of PDF inhibitors as antibacterial agents with reduced resistance. Considering that the predictivity of the model these molecules are good enough to be synthesized and tested for their activity to validate the model. ACKNOWLEDGEMENTS Computational studies were carried out in University Institute of Chemical Technology, University of Mumbai, Matunga, Mumbai-400019. The authors gratefully acknowledge support from the University Grants Commission (UGC), New Delhi under its DSA and COSIST programmes. IV thanks UGC for the award of senior research fellowship. REFERENCES 1. Giglione C, Pierre M and Meinnel T Peptide deformylase as a target for new generation, broad spectrum antimicrobial agents Molecular microbiology, 2000, 36(6),1197-1206 2. Johnson KW, Lofland D, Moser HE PDF inhibitors: An emerging class of antibacterial drugs. Current drug targets-Infectious Disorders 2005,5,39-52 3. Verma SK, Jat RK, Nagar N, A novel antibacterial target: peptide deformylase. Pharmacophore 2011,2(2),114-123 4. Jain R, Chen D, White RJ, Patel DV, Yuan Z Bacterial peptide deformylase inhibitors: A new class of antibacterial agents. Current Medicinal Chemistry 2005, 12,1607-1621 5. Becker, A, Schlichtings, I., Kabsch, W., Schultz, S. Wagner, A. Structure of peptide deformylase and identification of substrate binding site. Journal of Biological Chemistry 1998,11413-11416 6. Chen DZ, Patel DV, Hackbarth CJ, Wang W etal. Actinonin, a naturally occurring antibacterial agent is a potent deformylase inhibitor. Biochemistry 2000, 39, 1256-1262 7. Nguyen KT, Hu X, Colton C, Chakrabarti R. Characteriastion of human peptide deformylase: Implication for antibacterial drug design. Biochemistry 2003, 42, 9952-9958 8. Yuan Z, Trias J, White R. Deformylase as a novel antibacterial target Drug Discovery Today 2001, 6(18), 954-961 9. Chen D, Hackbarth C, Ni ZJ , Wu C, Wang W, Jain R, He Y etal. Peptide deformylase inhibitors as antibacterial agents: Identification of VRC3375 as a proline-3-alkylsuccinyl hydroxamate derivative by using an integrated combinatorial and medicinal chemistry approach . Antimicrobial Agents and Chemother. 2004, 48(1), 250-261 10. Hu X, Nguyen KT, Verlinde CLM, Hol WG, Pei D. Structure-based design of macrocyclic inhibitor for peptide deformylase. J. Med. Chem. 2003,46, 3771-3774 11. Boularot A, Giglione C, Petit S, Duroc Y etal. Discovery and refinement of new structural class of potent peptide deformylase inhibitors. J. Med. Chem. 2007, 50 (1), 10-20 12. Flipo M, Beghyn T, Charton J, Leroux VA. A library of novel hydroxamic acids targeting the metallo-protease family: Design, parallel synthesis and screening Bioorg. Med. Chem. 2007,15,63-76 13. Apfel, C., Banner, DW, Bur D, Dietz M., Hubschwerlen, HL, Locher H, Page M etal. Hydroxamic acid derivatives as potent peptide deformylase inhibitors and antibacterial agents. J. Med.Chem,2000,43, 2324-2331 14. SYBYL Molecular Modeling System ,version 6.6; Tripos Inc., St. Louis, MO 63144-2913 Table 1: Dataset of -Sulfonyl and sulfinylhydroxamic acid derivatives (O)n R3 S NHOH R1 R2 O Sr.No. R1 R2 R3 n IC50(E.coli PDF) µM Log1/IC50 1. Phenyl Propyl H 2 0.160 0.7959 2. Phenyl Butyl H 2 0.035 1.456 3. Phenyl Pentyl H 2 0.140 1.854 4. Phenyl Phenyl H 2 0.160 0.7959 5. Phenyl 2-furanyl H 2 0.094 1.027 6. Hexyl Butyl H 2 0.530 0.2757 7. Cyclohexyl Butyl H 2 0.230 0.6383 8. Benzyl Butyl H 2 0.190 0.7212 9. 2-Naphthyl Butyl H 2 0.023 1.638 10. 4-MeOC6H4 Butyl H 2 0.031 1.5086 11. 3-MeOC6H4 Butyl H 2 0.086 1.0655 12. 4-AcNHC6H4 Butyl H 2 0.011 1.9586 13. 4-BrC6H4 Butyl H 2 0.120 0.9208 14. Phenyl Butyl H 1 0.100 1 15. 4-MeOC6H4 Butyl H 1 0.099 1.004 16. 3-MeOC6H4 Butyl H 1 0.094 1.0269 17. 2-Naphthyl Butyl H 1 0.064 1.1938 18. 4-BrC6H4 Butyl H 1 0.210 0.6778 19. Phenyl Butyl OH 1 0.280 0.5528 20. Phenyl Phenyl H 2 0.016 1.7959 Table 2: Summary of CoMFA results Parameter Value r2cv 0.707 Number of components (Nc) 2 Standard error of predictions (SEP) 0.342 r2ncv 0.988 F 92.87 S (steric) 63.9 E (electrostatic) 36.9 r2pred 0.826 Table 3: Predicted PDF inhibitory activities for proposed inhibitors Predicted Log1/IC50 Predicted IC50 for PDF of E.coli (M) Compound I 0.9618 0.109 Compound II 1.2155 0.061 Compound III 1.3061 0.0494 Figure 1: Graph showing predicted activities vs. actual activities for the training set Predicted Activity Training Set 3 2.5 2 1.5 1 0.5 0 0 0.5 1 1.5 Actual Activity 2 2.5 Figure 2: Graph showing predicted activities vs. actual activities for the test set Predicted Activity Test Set 2.5 2 1.5 1 0.5 0 0 0.5 1 1.5 Actual Activity 2 2.5 Figure 3: Stereo view of CoMFA steric contour plot with most active compound 12 ( green polyhedra: sterically favored; yellow polyhedra: sterically disfavored ) Figure 4: Stereo view of CoMFA electrostatic contour plot with most active compound 12 ( blue polyhedra: electropositive groups favored ; red polyhedra: electronegative groups favored Figure 5: Stereo view of CoMFA steric contour plot with least active compound 6 ( green polyhedra: sterically favored; yellow polyhedra: sterically disfavored ) Figure 6: Novel proposed PDF inhibitors OH O NH NHOH O Compound I OH O O NH O Compound II NH NHOH OH O Compound III NHOH