* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download minerals!
Survey
Document related concepts
Transcript
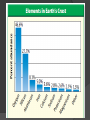
They’re everywhere! http://www.mii.org/ What is a mineral? A mineral: is naturally-occurring. ○ not manmade is an inorganic solid. ○ It has never been a part of a living thing. has a definite chemical composition. ○ It has specific elements that make up its compounds. ○ NaCl is always halite, which is table salt. It is a compound of sodium and chlorine. has an orderly arrangement of atoms. ○ Minerals are made of crystals, which have specific shapes. ○ All minerals are solids. Atom Patterns in Minerals Minerals are crystalline solids. The atoms of minerals are arranged in patterns that repeat. The Structure of Minerals A crystal is a solid in which the atoms are arranged in orderly, repeating patterns. A crystal system is a group of crystals that has a similar atomic arrangement. a similar overall crystal shape. http://www.minerals.net/MineralMain.aspx Crystal Systems of Minerals Hexagonal Six-sided crystals Examples: Emeralds Aquamarine Crystal Systems of Minerals Tetragonal Example: Zircon Cassiterite from Madagascar Apophyllite Crystal Systems of Minerals Cubic Examples: Halite, which is common table salt. Fluorite, which is an additive in toothpaste used to prevent cavities. Crystal Systems of Minerals Orthorhombic Example: Sulfur Topaz Crystal Systems of Minerals Monoclinic Example: Selenite gypsum Crystal Systems of Minerals Triclinic Very little symmetry Example: amethyst More on crystals . . . There are about 4000 different types of minerals on Earth. Crystals that develop in a tight space will have small, barely visible crystal shapes. Crystals that develop in open spaces will have more room to grow larger, visible crystal shapes. Crystals from Magma Hot, melted rock material that is under Earth’s crust is called magma. Magma is loaded with compounds that can form minerals. If magma cools slowly, the crystals have more time to form and can grow quite large. If magma cools quickly, smaller crystals form and individual crystals can’t be seen. Crystals from Solution Crystals can form when minerals dissolve in water and the water evaporates. http://news.nationalgeographic.com/news/2007/04/photogalleries/gi ant-crystals-cave/ Death Valley Salt fields were formed from halite that came out of solution as water evaporated. Devil’s Golf Course, Death Valley, California Mineral Compositions & Groups Ninety-two elements occur naturally in Earth’s crust. Most of Earth’s crust is composed of only 8 of these elements. Silicates are minerals that contain a compound of silicon and oxygen bonded together, usually with another element. Silicon and oxygen are the two most abundant elements in Earth’s crust.