* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Advanced Pharmacology-I (PHR5001) Introduction to Pharmacology
Polysubstance dependence wikipedia , lookup
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Plateau principle wikipedia , lookup
Theralizumab wikipedia , lookup
Psychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropharmacology wikipedia , lookup
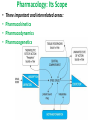
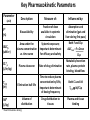
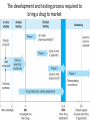
Advanced Pharmacology-I (PHR5001) Introduction to Pharmacology Dr. M G Azam Asstt. Professor Dept. of Pharmacy, NSU Pharmacology The word pharmacology comes from the Greek word for drug, pharmakon (means “an active principle”). Pharmacology is “knowledge about drugs”. It is the study of what biologically active compounds do in the body, and how the body reacts to them. Drug & Body • A drug is anything that affects the way an organism works. For now we only consider drugs which are used to cure a disease. • A disease is anything which affects the proper functioning of the body. It can be an infection, a genetic disorder, or the result of environmental conditions such as malnourishment, poisoning, or stress. • Engineers often find it easy to see the body as a factory. • Most of the work in our body is done by proteins. The body contains thousands of different kinds of proteins. The construction of each is determined by the DNA in the nucleus of each cell. DNA may be thought of as long strings of instructions which code for how each protein is too be built. Pharmacology • Pharmacology is the science of drugs, which deals with the detailed study of source, – chemical nature, – route of administration, – absorption, – distribution, – metabolism, – excretion, – pharmacological effects and – side effects of the drugs. Pharmacology: Its Scope • • • • Three important and interrelated areas: Pharmacokinetics Pharmacodynamics Pharmacogenetics Pharmacokinetics Pharmacokinetics is what the body does to the drug. The magnitude of the pharmacological effect of a drug depends on its concentration at the site of action : •Absorption •Distribution •Metabolism •Elimination e.g. Pharmacokinetics of Paracetamol : -rapidly & almost completely absorbed, orally attaining peak blood levels at 30-60 min; - 25%bound to plasma proteins, widely & uniformly distributed in the body; - extensively metabolized in the liver, primarily by glucuronide & sulfate conjugation into inactive metabolites which are excreted in urine; - has a plasma half life 2-3 hours . Pharmacodynamics Pharmacodynamics is what the drug does to the body. It is the interaction of drugs with cellular proteins such as receptors or enzymes to control changes in physiological function of particular organs. It covers : •Drug-Receptor Interactions •Dose-Response study •Signal Transduction : Mechanism of action, Pathways e.g. Pharmacodynamics of Paracetamol : -Paracetamol inhibits prostaglandin synthesis in the CNS. - It has less effect on COX in peripheral tissues which accounts for its weak anti-inflammatory activity. Pharmacodynamics Pharmacodynamics includes interaction between the drug and target cells or tissues and the body’s response to that interaction. : A) Effects of the drug: both beneficial & harmful effects • What does a drug do in the body? B) Mechanism of actions of the drug • How does a drug act in the body? Pharmacogenetics Area of pharmacology concerned with unusual responses to drugs caused by genetic differences between individuals. Responses that are not found in the general population but due to an inherited trait that produces a diminished or enhanced response to a drug. An individual's response to a drug depends on the complex interplay between environmental factors and genetic factors. Variation in drug response therefore may be explained by variation in environmental and genetic factors, alone or in combination. Differences in Enzyme Activity – Most common drug metabolizing enzyme family Cytochrome P450 (CYP) includes >30 isoforms Therapeutics Therapeutics deals with Use of drugs in living body for therapeutic purpose Therapeutics is the extension of the knowledge gained from medical pharmacology to the rational use of drugs (RUD) in the treatment of disease The goal of therapeutics is to achieve a desired beneficial effect with minimal adverse effects Pharmacotherapeutics • It deals with the practical application of drugs in the treatment and prevention of disease. • The way we use drugs to prevent and treat diseases • Vaccines prevent disease – Are the most cost-effective, most important medical development of the 20th century • Other drugs treat disease Basic principles of drug therapy provide a conceptual framework for deploying drugs with maximal efficacy while minimizing the risk of adverse effects. Drug Actions – Drug Interactions • Desired effect: Effect of drug in the body that was intended • Side effect: Additional effect on the body by the drug that was not part of the goal for that medication • Adverse reaction: One in which the body reacts to a drug in an unexpected way that may endanger a patient’s health and safety • Contraindication: Any special symptom or circumstance that indicates that the use of a particular drug or procedure is dangerous, not advised, or has not been proven safe for administration • Local effect: Response to a medication that is confined to a specific part of the body • Systemic effect: Generalized or widespread response to a drug by the body because it is absorbed into the bloodstream Some other definitions Effects (therapeutic effects) “The desired results of administration of a medication” "Therapeutic Window" /"Therapeutic Concentration Range" is the range of concentration over which the probability for therapeutic success is very high at only limited toxicity. Indications “The reasons for administering a medication or performing a treatment” Pharmacologic Profile: A description of all of the pharmacologic effects of a drug (e.g., effects on blood pressure, respiration, renal function, endocrine function, the central nervous system, etc.). Receptor: “A specific protein in either the plasma membrane or interior of a target cell with which the drug combines” Concept of Drug-Receptor Interactions Therapeutic and toxic effects of drugs result from their interactions with molecules in the patient. Most drugs act by associating with specific macromolecules in ways that alter the macromolecules' biochemical or biophysical activities The receptor concept has important practical consequences for the development of drugs and for arriving at therapeutic decisions in clinical practice. 1. Receptors largely determine the quantitative relations between dose or concentration of drug and pharmacologic effects. 2. Receptors are responsible for selectivity of drug action. 3. Receptors mediate the actions of both pharmacologic agonists and antagonists Agonists: Drugs that interact with a receptor to produce a biologic response are termed AGONISTS. An agonist has affinity for the receptor and efficacy. An ANTAGONIST is a drug that binds to a specific receptor, but the drug-receptor interaction does not lead to a biologic response. An antagonist has affinity for the receptor but low or zero efficacy For Example, Half -Life (T ½) • Half-life of a drug means the time in which the concentration or effects of the drug decline by one • The time needed for a drug's level in the blood stream to go down to one half its beginning level. In the simplest case, the body may be considered as a single compartment of a size equal to the volume of distribution (Vd). The time course of drug in the body will depend on both the volume of distribution and the clearance Half-life Peak serum concentration Drug serum Concen tration (mcg) ½ peak Time of Maximum Drug concentration Time Dose-Response Curve 1. Sub-effective dose 2. Ceiling effect, or maximal effect 3. Threshold effect and threshold dose 4. The plot shows a graded response dose-dependent effect Potency & Efficacy Potency is related to the size of the dose necessary to produce a certain effect. It is determined in part by the affinity of the receptor for the drug. Efficacy (or intrinsic activity) is related to the maximal effect obtained by the drug (i.e., when all receptors are bound to drug) ED50 is the dose required to cause a therapeutic effect (positive response) in 50% of a population TD50 or LD50 is the dose required to produce a toxic effect (or death in animal studies) in 50% of the studied population. For every drug there is a doseresponse curve for effectiveness and a doseresponse curve for toxicity Therapeutic Index is an indication of safety. The larger the ratio the safer the drug. Narrow therapeutic index drugs Warfarin Lithium Digoxin Phenytoin Gentamycin Amphotericin B 5-fluorouracil AZT (zidovudine) Key Pharmacokinetic Parameters Parameter (unit) F* (%) AUCp (uM.hr) CL*p (L/hr/kg) T1/2 (hr) Vd* (L/kg) Description Measure of: Influenced by: Bioavailability Fraction of dose available in systemic circulation Absorption and elimination (gut and liver during first pass) Area under the plasma concentration vs. time curve Systemic exposure; Important determinant for efficacy and safety Both F and CLp AUCoral = F x Dose CLp Plasma clearance Rate of drug elimination Metabolic/excretion rate, plasma protein binding, blood flow Elimination half-life Time to reduce plasma concentration by 50%; Important determinant of dosing frequency Both CL and Vd T1/2 Vd/CLp Volume of distribution Drug distribution to tissues Plasma and tissue binding * Need Intravenous dosing Drug Development Process • Discovery and formulation: Synthesis of a potential new drug molecule and an understanding of its interaction (mechanism) with the appropriate biologic targets. Repeated application of this approach leads to compounds with increased potency and selectivity • Preclinical evaluation: Relevant biologic effects, drug metabolism, and pharmacokinetic profiles and particularly an assessment of the relative safety of the drug must be characterized in animals before human drug trials can be started. • Clinical evaluation -Phases I-IV: With regulatory approval, human testing can then go forward in three phases before the drug can be considered for approval for general use. • Post-marketing surveillance: A fourth phase of data gathering and safety monitoring is becoming increasingly important. The development and testing process required to bring a drug to market