* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Presentation2n - Pheonix India
Survey
Document related concepts
Transcript
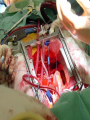
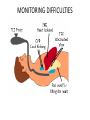
ANAESTHETIC CONSIDERATIONS IN CPB BY- DR SUCHIT KHANDUJA MODERATOR-PROF SURINDER SINGH DEFINITION • “CPB is the technique whereby blood is totally or partially diverted from the heart into a machine with the gas exchange capacity and subsequently returned to the arterial circulation at appropriate pressures & flow rates.” HISTORICAL ASPECTS • Legllois (1812) : “circulation might be taken over for short periods” • Dr.John Gibbon(Philadelphia) 1953 : “performed ASD repair with the aid of CPB for the 1st time with the survival of patient.” GOALS OF CPB • To provide a still & Bloodless Heart with blood flow temporarily diverted to an Extracorporeal Circuit that functionally replaces the Heart & the Lung GOALS OF CPB RESPIRATION Ventillation Oxygenation CIRCULATION TEMP. REGULATION (Hypothermia) Low blood flowso ed blood trauma ses Body Metabolism. COMPONENTS OF CPB • TOTAL CPB : Systemic venous drainage CPB Circuit External oxygenator heat exchanger External pump arterial filterSystemic circulation. • PARTIAL CPB : Portion of systemic venous return (Rt. Heart) CPB .Undiverted blood Rt. Atrium Rt. Ventricle Pul. Circulation Lt. Atrium & Lt. Ventricle Systemic Circulation. INTEGRAL COMPONENTS OF extracorporeal circuit • PUMPS OXYGENATOR Heat exchanger Arterial filter Cardioplegia delivery system Aortic/atrial/vena caval cannulae Suction/vent PATIENT ARTERIAL LINE FILTER RESERVOIR ROLLER PUMP OXYGENATOR HEAT EXCHANGER ROLLER PUMP • Most commonly used. • Uses Volume displacement to create forward blood flow. • Non Pulsatile Blood Flow • By compressing Plastic Tubing b/w Roller & Backing Plate • Properly set occlusion causes minimal haemolysis • Occlusion is 100% in cardioplegia &vent pumps • Each pump indepedently controlled by a rheostat • Larger tubing and lesser rotations cause minimal haemolysis • Resistance= resistance of tubing+oxygenator+heat excyhanger+filter+aortic cannulae+SVR • Usually line pr. depends on SVR and pump flow rate • Nl limit is 150-350 mm hg( >250 is seldom accepted) DISADVANTAGE of producing PULSATILE FLOW Bubble Formation Damage to Blood Components. ADVANTAGE : Improved Tissue Perfusion Better Preservation of Organ Function (Brain , Kidney) CENTRIFUGAL PUMP • Series of CONES that spin & propel blood forward by Centrifugal Force. • Safe • Reliable • Disposable • Simple to operate. CENTRIFUGAL PUMP • ADVANTAGE No back pressure when tubing is temporarily obstructed / kinked Doesn’t produce spatulated emboli from compression of the tubing Cannot pump large amt.of gas / gas emboli. Less blood trauma High vol. output with moderate pressures • DISADVANTAGE Inability to generate pulsatile flow Potential discrepancy b/w pump speed & actual flow generated. • Preferred over roller pumps in • Long-term CPB • In high-risk angioplasty patients • Ventricular assistance • Neonatal ECMO • Pressure-regulated pump • Operates under passive filling • After&pre-load sensitive • Pump-chamberof polyurethane+peristaltic pump • Not yet fully evaluated OXYGENATOR • Where O2 & CO2 Exchange takes place. • Two Types : BUBBLE OXYGENATOR MEMBRANOUS OXYGENATOR BUBBLE OXYGENATOR • Gas exchange by directly infusing the gas into a column of systemic venous blood. • A) OXYGENATING CHAMBERS : bubbles produced by ventilating gas through diffusion plate into venous blood column • CO2 bubble & oxygen plasma • Larger the No. of Bubbles ; Greater the efficiency of the oxygenator. • Larger bubbles improve removal of CO2 , diffuses 25 times more rapidly in plasma than O2 • Smaller bubbles are very efficient at oxygenation but poor in co2 removal DEFOAMING CHAMBER • Defoaming of frothy blood. • Large surface area coated with silicone • This es the Surface Tension of the bubbles causing them to burst. BUBBLE OXYGENATOR • • • • ADVANTAGE Easy to assemble Relatively small priming Volumes Adequate oxygenating capacity Lower cost. • • • • • DISADVANTAGE Micro emboli Blood cell trauma Destruction of plasma protein due to gas interface. Excessive removal of CO2 Defoaming capacity may get exhausted with time. MEMBRANOUS OXYGENATOR • Gas exchange across a thin membrane • Eliminates the need for a bubble-blood contact & need for a defoamer; so more physiological. • Blood damage is minimum • Ideal for perfusions lasting for >2-3 hours. • 2 types of membrane: • SOLID: Silicone • MICROPOROUS: polypropylene,Teflon &polyacrylamide MEMBRANOUS OXYGENATOR ADVANTAGE • Can deliver Air-O2 mixtures. • Hemolysis • Protein desaturation • Post-op bleeding • Better platelet preservation. DISADVANTAGE • Expensive • Large priming volume • Prolonged use pores may get blocked. CIRCUITS † Drains Venous Blood by gravity into oxygenator & returns the oxygenated blood under pressure to the systemic circulation. VENOUS DRAINAGE • Systemic venous blood (Rt.Heart)Oxygenator by Direct Cannulation of SVC & IVC (Bicaval Cannulation) thru RA & joined to create a single drainage channel. Single cannula into RA thru RA appendage. • Blood flow Oxygenator (Gravity) • Height Difference B/w Venacavae & Oxygenator > 20-30 cm. Complications • arrhythmia • bleeding • ivc/svc tear • cannula malposition • low return • inadequate height • malposition • kink,clamp,air lock Size of cannula Adults Children SVC 28G 24G IVC 36G 28G TUBINGS IN THE CIRCUIT • Non thrombogenic , Chemically Inert to prevent clotting Trauma to blood elements Protein Denaturation • Smooth Internal Finish • Non Reactable Internal Surface • Durable to withstand high pressure & use of Roller pump • Made of • PVC • Polyurethane • Silicone • I.D . Ranges from 3/16- 5/8 inches • HEPARIN BONDED CIRCUITS ARE AVAILABLE Disadvantages of plain circuits • Activation of platelets/coagulation factors • Post-op consumptive coagulopathyimmune reactions • More spallation Heparin coated circuits are • More hemo compatible • Cause less activation of platelets/white cells • Reduce heparin demand INTRACARDIAC SUCTION • Blood will enter the heart Coronary venous Return Retrograde flow in AR. Bronchial Arteries CARDIOTOMY SUCTION • Spilled Heparinised Blood is Scavenged & returned back to patient. • Handheld Suckers are used to return this blood. VENTRICULAR VENTING • LV Venting done to • Keep the operative field clear • Maintain Low LA & Pul.Venous Pressure • Remove air from Cardiac Chamber. •Blood from LV Reservoir Bag RESERVOIR BAG • Collects the blood from VENOUS DRAINAGE & CARDIOTOMY SUCTION DRAIN PASSIVELY Reservoir Bag Oxygenator Heat exchanger Arterial Filter Patient. • Volume in the bag should not be allowed to empty to prevent massive emboli. ARTERIAL RETURN • Ascending Aorta just proximal to Innominate Artery. • Femoral Artery in Dissecting Aortic Aneurysm For Reoperation Emergency • Problems of Femoral Cannulation : • Sepsis • Formation of False Aneurysm • Development of Lymphatic Fistula. ARTERIAL CANNULA • Is the Narrowest part of the circuit. • Should be as Short as possible. • As Large as the diameter of vessel permits. Complications • Difficult Cannulation • Intramural Placement • Air embolism • Dislodgement of Cannula • Dissection • Arch Vessel Cannulation • Back Wall Injury • MICROPORE FILTERS: • Remove Particulate Matter (Bone , Tissue , Fat , Blood Clots etc.) • Pore Size : 30 – 40 • ULTRAFILTRATION : • Remove the excess fluid from the CPB. PRIME FLUID • Ideally close to ECF. • Whole Blood NOT used : • Homologous Blood Syndrome. • Post Perfusion Bleeding Diathesis • Incompatibility Reactions. • Demand on Blood Banks. • Addition of Priming Fluid HEMODILUTION. COMPOSITION OF PRIME : • Balanced salt soln. RL • Osmotically active agent (Mannitol, Dextran 40 , Hexastarch) • NaHCO3 • KCl • Heparin 1250 ml 100 ml 50ml 10ml 1ml PRIMING • Heme, nonheme • Decreases viscosity so better flow • Attenuates increased viscosity by hypothermia • Alters pharmacodynamics and kinetics of drugs • Decreases Hb but improves O2 delivery • Lowest acceptable value 8g/dl • Prediction of initial haematocrit during CPB Predicted Hct = volume – EBV • Infants • Children • Adult (male) • Adult (female) Pt. RBC volume before CPB / Pt. EBV + CPB prime 80-85 ml/kg 75ml/kg 70ml/kg 65ml/kg – 1U packed cells = 0.7 x 350 = 245ml – IU whole blood = 0.4 x 350 = 140ml • Amount of priming fluid • CVX CPCV = Pt. BV X PCV + PV X PCV • PT.BLOOD VOL. x PT. HEMATOCRIT = TARGET HCT X(PRIME VOL. + PT. BLOOD VOL.) PATHOPHYSIOLOGY OF CPB • THREE MAJOR PHYSIOLOGICAL ABERRTIONS ARE: 1.LOSS OF PULSATILE FLOW 2.EXPOSURE OF BLOOD TO NON-PHYSIOLOGIC SURFACES & SHEAR STRESSES. 3.EXAGGERATED STRESS RESPONSE. CIRCULATORY SYSTEM • SVR : Initial Phase SVR i. Blood Viscosity 20 to Hemodilution. ii. Vascular Tone d/t dilution of circulatory catecholamines As CPB BP , d/t SVR a) Actual in Vascular C/S area d/t closure of portions of microvasculature. b) Catecholamines c) VC d/t hypothermia. • Cardiac output : flow rate at 2.2-2.4 l/m2/min at 370c. • BP : 0-70 mm Hg. • Venous tone : Close to zero PULMONARY EFFECT • Activated neutrophils (elastase &lysosomal enzyme ) accumulate within the lungs during CPB. • Pul. Venous Pressure , 20 to LAP , es the risk of Pul.Interstitial Edema. * After CPB Pul.Compliance falls & Airway Resistance leading to Work of Breathing. CNS CHANGES • Embolic phenomena : –Air –Preexisting thrombi –Platelet & leucocyte aggregate –Fat globules • Hemodilution –> mild cerebral edema • CBF when MAP es <40mmHg during CPB RENAL EFFECT • MICRO EMBOLI • Vasoconstrictors • Ppt. of Plasma Hb in Renal tubules U.O. • Long term ace inhibitor therapy can result in decline in glomerular filteration pressure HEMATOLOGIC EFFECT • RBC : become stiffer & less distensible –Exposed to Non-physiologic surfaces – Hemolysis d/t high flow rates • WBC : Marked in PMN • PLATELETS : aggregation & dysfunction thrombocytopenia. HEMATOLOGIC EFFECT • PLASMA PROTEIN :Denaturation – Altered enzymatic function – Aggregation of platelets – Altered solubility characteristics – Release of lipids – Absorption of denatured proteins into cell membranes. NEUROENDOCRINE RESPONSE TO CPB: • Serum Catecholamines : –Both ADR & NA –D/t reflexes from Baroreceptors & Chemoreceptors in the Heart & Lungs when the organs are excluded from circulation. • ADH,Cortisol , Glucagons & GH are INDUCTION OF ANAESTHESIA • Choice depends on haemodynamic status 1. High dose opioid anesthesia 2. Total intravenous anesthesia 3. Mixed iv/inhalational agent anesthesia • High dose opioid • Fentanyl -50-100mic/kg,sufentanil 15-25mic/kg ADV-Faster extubation Disadvantage1. Prolonged respiratory depression 2. Chest wall ridgidity 3. Patient awareness 4. Inability to control hypertensive response Total iv anaesthesia • Propofol 1-2 mg/kg with infusion of 50100mic/kg/min • Remifentanil 0-1mic/kg bolus followed by .251mic/kg/min • TCI may be used Mixed IC/Inhalational anesthesia • Interest grew after studies on protective effect of volatile agents on myocardium • Propofol,thiopentone,midazolam may all be given • Opioid given in smaller dosages with inhalational agent at .5-1.5 MAC • Isoflurane,sevoflurane and desflurane used • N2O not used because of its tendency to expand bubbles in intravasular compartment during CPB • Isoflorane.desflorane and sevoflorane cause dose dependent vasodilation. • Also lead to ischaemic preconditioning. • N20 usually avoided . • Radial artery is cannulated. • Contralateral femoral also used as conduit. • Cvp catheter or PA catheter or both introduced. • Bladder catheter,temp. probe and TEE probe positioned. PRE-CPB Two stages – High level of stimulation • Skin incision, sternal split, sternal spread, aortic dissection cannulation. • Increase HR, BP, ischemia, dysrhythmias, HF – Low level of stimulation • Preincision, Radial artery harvesting, dissection, CPB venous cannulation. • Decrease HR, BP, ischemia, dysrhythmias LIMA • • • • • All injection ports should be accessible Monitoring lines should be well secured Confirm zero of all transducers Evaluate cardiac status by TEE (placed before heparin). Once patient stabilized – ABG, ACT, BSL, Serum electrolytes • Antibiotics • Antifibrinolytics – Aprotinin, EACA, Transexamic acid • Pre-incision, sternal split – Supplemental - Narcotics, relaxants, hypnotics, inhaled agents. – Ensure adequate depth of anaesthesia. • Redo case – Lateral CXR provides a clue to potential problems – Longer time required than routine – Femoral vessels to be prepared – External defibrillator – Adequate volume replacement – crystalloids, colloids, blood and blood products. HEPARIN • Jay Mclean-1916, William Howell • N-sulfated-D-Glucosamine& L-iduronic acid • strongest acid, anionic, negative charged • Heterogenous compound, mol wt 5000 – 30000 (most chains 12000-19000). • UFH dose should not be specified by weight but by units. • 1 USP of heparin activity is the quantity that prevents 1 ml of citrated sheep”s plasma from clotting for 1 hr after addition of calcium. • Standard heparin is UNFRACTIONATED HEPARIN (UFH ). • Heparan • found in CT, contains more of glucuronic acid >20% Nacetylation. • Abundant in tissues rich in mast cells • liver, lungs, intestines • skin, lymph nodes, thymus lesser sources. • Two sources • Bovine lung • Porcine intestinal mucosal ( most commercial prep , 40000 lbs yield 5kg heparin) • Pharmacokinetics & dynamics: • 3 compartment model describes heparin kinetics • Rapid initial clearance from endothelial cell uptake • Saturable clearance seen in lower doses due to uptake by RES & its endoglycosidases, endosulfatases & uptake into monocytes. • Exponential decay seen at higher doses due to renal clearance via tubular secretion. • Metabolism: • 50%- RES • 50%- Renal elimination • Actions: • Exerts its actions via AT-III which inhibits thrombin, IXa, Xa. • UFH accelerates the formation of thrombin-AT complex 2000 X, Xa-AT complex 1200X • LMWH preferentially inhibits Xa HEPARIN RESISTANCE/ ALTERED HEPARIN RESPONSIVENESS: • Pts previously receiving heparin exhibit tachyphylaxis, diminished response to full anticoag doses of UFH for CPB. Risk Factors: • Elderly/ neonates • IABP • Previous heparin therapy • Shock • OCP/ Pregnancy • STK • Thrombocytosis • Infective endocarditis • Congenital AT-III deficiency • Ventricular aneurysm with thrombus • Consumptive coagulopathy • Hemodilution • Dose: • 3-4 mg/ kg • 300-400 u/ kg • given in central vein or directly into RA • use HDR • always confirm with ACT UFH chelates Ca, large bolus- decline in BP due to decrease in SVR & preload. Immunologic effects-30-50% pts of cardiac surgery have heparin Abs by the time of hospital discharge • Arterial sample in 3-5 min • Give additional heparin as needed to maintain ACT >300 s in normothermic and >400 s in hypothermic CPB • Monitor ACT every 30 min or more frequently if pt.is heparin resistant • If ACT goes <300 s give additional 50 u/kg heparin ACTs • <180 s - life threatening • 180-300 s -highly questionable • >600 s –risky and unwise • Individual anticoag response to heparin varies , hence measurement of individual anticoag response to heparin for CPB is warranted. Usually heparin effect is measured and not its plasma levels. TREATMENT – Additional heparin – AT-III concentrate (1000 u increases AT-III levels by 30% – rhAT trials on (75 U/ kg) – FFP ( risk of infection transmission, reserved for rare refractory cases) HEPARIN REBOUND • pts develop clinical bleeding assoc with prolongation of coagulation times due to reappearance of circulating heparin. CAUSES – late release of heparin sequestered in tissues – delayed return of heparin to circulation from extracellular space via lymphatics – clearance of antagonist an unrecognized endothelial – more rapid clearance of protamine to heparin. • Incidence-50% • Can occur as soon as 1 hr after prota adm heparin TREATMENT: • Clinical bleeding does not always accompany heparin rebound. • If + -additional supplemental protamine. • Larger initial doses may decrease likelihood but risk of adverse cardiovasc sequelae & anticoag effects of protamine. • Use HDR to calculate heparin. HIT • Heparin normally binds to platelet memb at GP Ib and aggregates normal platelets by releasing ADP. • Type-I – moderately reversible – Prolongation of BT • Type-II – Occ severe & progressive thrombocytopenia (<1 lac) – Accom by severe fatal thrombosis – Drop in platelet count > 30-50% over several days in a pt receiving or finished receiving heparin. • Heparin dependent Abs usually IgG present, lower titres during therapy but rise once therapy ceases. DIAGNOSIS: • Incidence 1-3% • Dose related but can occur even with heparin flush or heparin bonded intravascular catheters. • Usually 3-15 days after heparin but can occur within hours in a pt previously exposed to heparin • Decrease in platelet counts • Serotonin release assay- pt plasma + donor platelets containing radiolabelled serotonin + heparin. • ELISA for Ab to hep-PF4 complex. Treatment • PC not indicated • Discontinue heparin • Start alternative anticoag • Surgery for thrombosis • Aspirin, ticlopidine, dipyridamole block adhesion and activation and PF4 release • Delay surgery to wait for Abs to regress • Plasmapheresis • Heparin substitutes HEPARIN SUBSTITUTES CANNULATION • Aortic cannula first – Maintain SBP to 90-100 – MAP 60 –80 – Excessive lowering – damage to posterior wall – Largest possible size • Check line pressures • Sandblasting effect • Coanda effect Complications • Difficult Cannulation • Intramural Placement • Air embolism • Dislodgement of Cannula • Dissection • Arch Vessel Cannulation • Back Wall Injury • Venous Cannulation – Single stage • Atrial • Bicaval – Two stage • Atriocaval – Peripheral • size is important Complications • arrhythmia • bleeding • ivc/svc tear • cannula malposition • low return • inadequate height • malposition • kink,clamp,air lock HYPOTHERMIA • • • • Decreases BMR VO2, VCO2 Provides organ protection and safety margin Decreases excitatory NT (glutamate) release Decreases rate of enzymatic reaction • Q10 - change in reaction rate for 100C (2-3) • Can use non sanguinous primes and lower flows • Increases SVR, PVR • Decreases blood trauma • Decreases blood flow to all tissues but also req. • Decreases HR • Dysrhythmia occur – Nodal, VPC, AF, VF, blocks, asystole • Decreases ventilation • Left ward shift of ODC • Increases dead space – no effect on gas exchange • Increases renal vascular resistance • Decreases renal blood flow • Decreases tubular reabsorption • Urine flow may be increased • Hepatic blood flow decreased • Decreased metabolic and excretory liver function • Marked hyperglycemia – decrease insulin increased catechol • Affects coagulation by platelet dysfunction and inhibition of coagulation factor. Mild 32 –350C Moderate - Deep 18-260C Profound - 26-310C <180C • Cooling / rewarming facilitated by increasing pump flow rates and dilators. • Gradient < 5-100C, never exceed 400C. NORMOTHERMIC CPB • Warm cardioplegia – Better myo substrate use – (L) ODC shift avoided – Diastolic arrest produces greatest reduction in MVO2 – Continuous CP attenuates reperfusion injury • No need for rewarming • Earlier extubation • Lower SVR so higher flow rates, vasoconstrictors Ideal temperature – indeterminate • Tepid CPB 32-340C • Terminate CPB 34-350C MEAN ARTERIAL PRESSURE • maintain 70-90 @ normothermia • 50-70 mm Hg 30-32°C • 30-40 mm Hg @ <30°C • Higher pressures- increased non coronary collateral flow • Maintain adequate flows • SVR– increased by phenylephrine, noradr – decreased by NTG, SNP, anaesthetics Sub-groups needing higher pressure • Severe atherosclerosis • Advanced age • Hypertension • Diabetes CPB INFLAMMATORY RESPONSE • Blood contact with non endothelial surface • Complement system, monocyte- macrophage system, cytokines, endotoxins, free radicals,metalloproteinases • Systemic inflammatory response to bypass MONITORING PATIENT • ECG • CVP/ PAC • Arterial/ Perfusion pr • Temperature • Coagulation-ACT,TEG • Urine output • SpO2 ,EtCO2 • Se electrolytes • ABG • Hb, Hct • BSL • TEE • EEG, BIS • SjvO2 PUMP Inline blood gas monitoring Venous oximetry Line pressure Temperature monitoring Flow Reservoir volume Bubbles CARDIOPLEGIA Route • antegrade aortic root, ostial maintain root pressure 50-100 mm Hg • retrograde coronary sinus maintain coronary sinus pressure 40-60 mm Hg Temp • warm • tepid • cold Interval • continuous Vehicle • blood • crystalloid • • • • total dose is 20-30 ml/ kg target myocardial temp. is 10-15ºC repeat every 20-30 min dose is usually ½ the induction dose with ½ the potassium conc. of the induction soln. AIMS OF CARDIOPLEGIC ARREST • induce arrest as quickly as possible • provide oxygenation • maintaining cellular integrity by maintaining Na-K ATPase • provide energy substrates for metabolism • maintain osmolarity to prevent cellular edema • possess buffering capability,oxygen free-radical scavenging capacity (best by blood ) • hypothermia helps in decreasing oxygen demand ULTRAFILTRATION • hydrostatic pressure • increased by increasing perfusion pressures or by applying vacuum on the effluent side of the memb • pore size is imp (10-35 A°) (20000 Da) • Alb, Hb, fibrinogen, blood cells remain back • MUF – after separation from CPB – blood from aortic cannula thru hemoconc to RA • CUF – performed during rewarming – volume removed is based on volume in CPB circuit – Limited in pediatric pt. (<10kg,small intravasc vol) & stopped once CPB is off • High Volume Zero UF – modification of CUF – UF volume is replaced with equal volume of crystalloid Advantages: • removal of free water • preservation of hemostasis • increases Hb,plat counts, fibrinogen, albumen • removes proinflammatory mediators-complements, TNF, IL-1, IL-6,IL-8 • removal of C-3a-decreases PVR, improves oxygenation, faster extubation • improves post-CPB hemodynamics- lower HR, inc SBP, higher cardiac index, better diastolic compliance • decreases cerebral edema • improves renal function post CPB WEANING FROM CPB C V P Cold Ventilation Predictor Conduction Visualisation Pressure Cardiac output Vapourizer Pressors Cells Volume expander Pacer Calcium Potassium Coagulation Protamine PROTAMINE • • • • • • Meischer 1868, Hagedorn & colleagues 1936 contains many positive charges, nearly 2/3rd arginine Chargaff & Olson- neutralizing drug salmon milt Stable without refrigeration for several weeks Available as sulfate & chloride salts ( Chloride has more rapid onset of action) Actions: • Formation of complexes with sulfate groups of heparin form the basis for antidote effect • Neutralizes AT effect of heparin far better than anti Xa effect, hence poor ability to neutralize LMWHs Advantages: • • • • • • • • removal of free water preservation of hemostasis increases Hb,plat counts, fibrinogen, albumen removes proinflammatory mediators-complements, TNF, IL-1, IL-6,IL-8 removal of C-3a-decreases PVR, improves oxygenation, faster extubation improves post-CPB hemodynamics- lower HR, inc SBP, higher cardiac index, better diastolic compliance decreases cerebral edema improves renal function post CPB • Recommended doses to neutralize heparin vary widely • lot of controversy regarding optimal prota-hep ratio, dose to prevent rebound & whether to calculate prota dose based on total amt of hep given or amt remaining in the pt. • Normally 1.3-1.5 X heparin doses, 75% given foll CPB & 25% foll reinfusion of pump blood • Best to use protamine titration tests GUIDELINES FOR USE: • Add to 50 ml clear infusion & adm infusion over 10-15min • Additional doses of undiluted prota given @20mg/ min • Slow adm decreases Type I & Type III adverse reactions but Type II can occur at any delivery rate • No screening test in DM, risk is only 0.6% • In pts with fish allergy skin testing is predictive,give 1mg prota diluted in 50ml over 10min & if no adverse response give full dose • In pts with prior reaction to prota skin testing, RAST, ELISA appropriate, test dose as before, use prota alternatives Adverse Reactions TREATMENT • • • • • • • Slow adm limits Type III since large complexes do not form Stop prota infusion Stop all cardiodepressant drugs IV fluids, Calcium Antihistaminics Hydrocortisone/ Aminophylline Adm of heparin bolus in an attempt to decrease hep-prota complex size • Ionodilators- Milrinone, Isoproterenol • Avoid rechallenge with prota • Reinstitute CPB TERMINATION FROM BYPASS • Ventilation has been re established. • Venous return to pump decreased by clamping the line. • If cardiac performance non optimal then additional blood from pump can be taken. • When BP,CO,Preload optimal arterial pump stopped,and venous canula removed. • Aortic canula not to be removed until test dose of protamine given. OFF PUMP CORONARY ARTERY BYPASS SURGERY • OPCAB accounts fot 20-30% of all CAB surgeries. • Performed through median sternotomy. • Bypass grafts include right and left mammary arteries,saphenous veins,and radial arteries. • Special sternal retractors used. • Fixation devices stabalise the area of heart. • Verticlisation of apex done. • Surgical visualisation with help of blower. • Distal anastomoses then performed. ANAESTHETIC CONSIDERATIONS • Revolve around haemodynamic perturbations. • Surface stabaliser and apical displacement device associated with dec. CO,Sv and MAP. • The above can also lead to distortion of mitral valve annulus. • Rewarming not done. MONITORING • • • • Standard ASA Arterial Catheter Central Venous A Ischemia Monitoring - EKG ST trend - PA Catheter? - TEE? MONITORING DIFFICULTIES • Haemodynamic changes treated with volume expansion. • Blood transfusion for high risk patients. • Hypotension can be treated with inotropes,volume expansion. • NTG to be used cautiously. • Worsening haemodynamic status or ecg changes warrrant insertion of intracoronary stents. • Conversion of OPCAB to CABG with CPB an option. ADVANTAGES OF OPCAB • There are several advantages to beating heart surgery from the anesthesiologist's perspective: • The patient is extubated early, is not severely anemic, is awake and breathing on his/her own. • There are no cannulation sites in large vessels which can potentially bleed. • Less hemodilution. • Coagulopathies are uncommon. OTHERS… • • • • • • Shorter postoperative hospital stays. Shorter time with ventilatory support. Less blood loss and need for transfusions. Less likelihood of low output syndrome. Reduced systemic inflammatory response. Fewer arrhythmia and neurologic postoperative complications. • Potential cost savings. THANX!!