* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ANTIVIRAL AGENTS
DNA-encoded chemical library wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of HIV-protease inhibitors wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
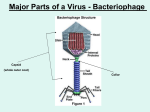
ANTIVIRAL AGENTS 1 Introduction to Virus Virus is a small (20-30 nm) infective agents which is an obligate intracellular parasite It contain either double- or single-stranded DNA or RNA enclosed in a protein coat called a ‘Capsid’ A virus depends upon host cell for its metabolic processes, such as synthesis of proteins and DNA It cannot replicate on its own 2 Introduction to Virus The virus must attach to and enter a host cell It then uses the energy of host cell to synthesize protein, DNA and RNA The free living virus particle (i.e. outside its host) is termed as Virion The life cycles of viruses are intimately associated with those of their host cells, hence it is difficult to find agents that selectively inhibit virus replication without damaging human cells Most drugs can not distinguish between viral function and host cell function, hence cause toxicity to both 3 Types of Virus with examples DNA viruses: poxviruses (smallpox), herpesviruses (chickenpox, shingles, cold sores), adenoviruses (sore throat, conjunctivitis) and papillomaviruses (warts) 4 Types of Virus with examples RNA viruses: orthomyxoviruses (influenza), paramyxoviruses (measles, mumps), rubella virus (German measles), rhabdoviruses (rabies), picornaviruses (colds, meningitis, poliomyelitis), retroviruses (acquired immunodeficiency syndrome (AIDS), T cell leukaemia), arenaviruses (meningitis, Lassa fever), hepadnaviruses (serum hepatitis) and arboviruses (various fevers, e.g. yellow fever). 5 Viral Cell Replication Major Steps Involved in Replication are: 1. 2. 3. 4. 5. 6. 7. Adsorption and penetration into host cell Uncoating of viral nucleic acid Synthesis of regulatory proteins Synthesis of RNA or DNA Synthesis of structural proteins Assembly of viral particles Release from host cell 6 Replication cycle of DNA virus 1. 2. 3. 4. 5. 6. 7. 8. Attachment Membrane fusion Release of viral DNA through nuclear pores Transcription of viral mRNA Synthesis of viral proteins by host cell’s ribosomes Replication of viral DNA by viral polymerases Assembly of virus particles Budding and release of progeny virus 7 Replication cycle of RNA virus 1. 2. 3. 4. 5. 6. 7. 8. 9. Attachment Endocytosis Influx of H+ through M2 protein Fusion of the viral envelope with the endosomes membrane and entry of viral RNA into the nucleus Synthesis of viral mRNA by viral RNA polymerase Translation of viral mRNA by host cell’s ribosomes Replication of viral RNA, using viral RNA polymerase, via cRNA replicative form Assembly of virus particles Budding and release of progeny virus 8 9 Antiviral Agents Key characteristics of antiviral drugs: Able to enter the cells infected with virus Able to interfere with viral nucleic acid synthesis and/or regulation Able to interfere with ability of virus to bind to cells Some drugs stimulate the body’s immune system Best responses to antiviral drugs are in patients with competent immune systems 10 Antiviral Agents- CLASSIFICATION [1] Anti-Herpes: • Acyclovir • Valacyclovir • Ganciclovir • Penciclovir • Cidofivir • Fomivirsen • Idoxuridine • Trifluridine • Docosanol [2] Anti-Influenza: • Amantadine • Rimentadine • Oseltamivir • Zanamivir [3] Anti-Hepatitis: • Adefovir • Ribavirin • Lamivudin • Palivizumab [4] Anti-Retroviral: • Zidovudine • Stavudine • Didanosine • Lamivudine • Zalcitabine • Efavirenz • Nevirapine • Delavirdine • Tenofovir • Saquinavir • Indinavir • Nelfinavir • Lopinavir 11 12 ANTI-HERPES VIRUS AGENTS Acyclovir Valacyclovir Ganciclovir Penciclovir Cidofivir Fomivirsen Idoxuridine Trifluridine Docosanol 13 Anti-Herpes virus agents Herpes simplex virus type 1 (HSV-1) causes diseases of the mouth, face, skin, esophagus or brain. Herpes simplex virus type 2 (HSV-2) causes infections of the genitals, rectum, skin, hands or meninges. Both cause serious infections in neonates. 14 Acyclovir and Valacyclovir Nature Mechanism of Action Therapeutic use Adverse Effects Acyclovir is an acyclic guanine nucleoside analog Valacyclovir is the ester prodrug of acyclovir Selective inhibition of viral DNA synthesis by interaction with two viral proteins: HSV Thymidine kinase (TK) HSV DNA polymerase Does not affect host DNA synthesis Herpes Simplex Virus (HSV) Infection: Primary herpetic gingivostomatitis Genital herpes Mucocutanious HSV infection (prophylaxis and treatment) Herpetic keratoconjunctivitis (ophthalmic formulation) Verisella Zoster Virus (VZV) Infection: Rash, fever, cutanious lesions Cytomegalo Virus (CMV) Infection: CMV retinitis Topical: mucosal irritation and transient burning when applied to genital lesions. Oral: nausea, diarrhea, rash, headache I.V.: severe renal insufficiency, nephrotoxicity and CNS side effects 15 16 Cidofovir Nature Mechanism of Action Therapeutic use inhibits viral DNA synthesis by terminating chain elongation metabolized by cellular enzymes to its active diphosphate form which competitively inhibit: dCTP a substrate for viral DNA polymerase (of both HSV & CMV) A phosphocholine metabolite serves as an intracellular drug reserviour Adverse Effects cytidine nucleotide analog Approved to be used in CMV retinitis in HIV infected patients Synergistic in combination with Ganciclovir or Foscarnet Acyclovir resistant Mucocutanious HSV infection (topical gel) Treatment of anogenital warts (topical gel) Foscarnet resistant CMV infection Adenovirus disease in transplant recipient BK virus nephropathy in renal transplant patient Nephrotoxicity Proxymal tubular dysfunction including proteinurea, azotemia, glucosurea Cycloplegia due to Occular hypotony Topical application: burning, pain, pruritis Considered as potential human carcinogen 17 Famciclovir and Penciclovir Nature Mechanism of Action Therapeutic use Adverse Effects Famciclovir is an ester prodrug of penciclovir Penciclovir is an acyclic guanine nucleoside analog Triphosphate form competitively inhibits viral DNA polymerase Inhibit DNA chain elongation (no chain termination) Oral famciclovir, topical penciclovir, and intravenous penciclovir are approved for HSV and VZV infections Treatment of genital herpes Reduce HSV recurrences in HIV-infected persons Chronic HBV hepatitis Oral famciclovir: Headache, diarrhoea, urticaria, rash, confusion Penciclovir is mutagenic at high concentrations 18 Fomivirsen Nature Phosphorothioate oligionucleotide Mechanism of Action Complimentary to m-RNA sequence of CMV, hence inhibit its replication Inhibit viral binding to cell Therapeutic use Adverse Effects Active against CMV strains resistant to ganciclovir, foscarnet, and cidofovir Given by intravitreal injection for patients unresponsive to other therapies for CMV retinitis Occular: irrititis, cataract, increased IOP Increased risk of inflammatory reactions 19 Foscarnet Nature Mechanism of Action Therapeutic use Reversibly and noncompetitively blocks the pyrophosphate binding site of the HSV DNA Polymerase Also interact directly with HIV Reverse Transcriptase Thus inhibits viral nucleic acid synthesis Adverse Effects Inorganic pyrophosphate analog CMV retinitis (i.v.) CMV pneumonia and viraemia Ganciclovir and acyclovir-resistant HSV and VZV infections Genital Ulceration Nephrotoxicity Acute tubular necrosis Nephrogenic diabetes insipidus Highly ionized at physiological pH, and metabolic abnormalities are very common, including: hypocalcemia, hypophosphatamia, hypomagnesemia, and hypokalemia Thrombophlebitis ECG changes 20 21 Ganciclovir and Valganciclovir Nature Ganciclovir is an acyclic guanine nucleoside analog Valganciclovir is the ester prodrug of ganciclovir Mechanism of Action Triphosphate form competitively inhibit DNA chain Therapeutic use Adverse Effects Chronic suppression of CMV retinitis (i.v.) Intraocular sustained-release ganciclovir implant (VITRASERT) is more effective in supressing Retinitis Benificial in infants with congenital CMV disease CMV infections in Organ transplant recipients HSV keratitis (ophthalmic gel) Myelosuppression (Higher risk with Zidovudin) Neutropenia (Treated with Recombinant G-CSF) Eosinophilia Thrombocytopenia GI disturbances Anemia 22 Doscosanol Nature Therapeutic use Long-chain saturated alcohol FDA approved as a 10% OTC cream for the treatment of recurrent orolabial herpes Topical treatment within 12 hours of symptom initiation is only effective 23 Idoxuridine Nature Mechanism of Action Iodinated thymidine analog Inhibits the in vitro replication of various DNA viruses (HSV and Pox virus) Triphosphate inhibits viral DNA synthesis Lacks selectivity between host cell and viral cell Therapeutic use Approved only for topical treatment of HSV keratitis Adverse Effects Pain Pruritis Inflammation Edema involving the eye 24 Trifluridine Nature Fluorinated pyrimidine nucleoside Mechanism of Action Monophosphate irreversibly inhibits thymidylate synthase Triphosphate competitively inhibits thymidine triphosphate incorporation into DNA Inhibit DNA synthesis Therapeutic use FDA approved for treatment of keratoconjunctivitis and keratitis owing to HSV types 1 and 2 Adenovirus infection Adverse Effects Discomfort and edema upon instillation Hypersensitivity reactions and irritation (Rare) 25 ANTI-INFLUENZA VIRUS AGENTS Amantadine Rimentadine Oseltamivir Zanamivir 26 27 Amantadine and Rimantadine Nature Mechanism of Action Therapeutic use Inhibit viral uncoating as well as viral assembly Interfere with function of M2 protein of Influenza A virus, which act as ion channel for viral uncoating Adverse Effects Both are tricyclic amines Rimantadine is a methyl derivative of Amantadine Both are effective for the prevention and treatment of Influenza A infection Seasonal prophylaxis of Influenza A Treatment of Hepatitis-C in combination with Interferon Amantadine: treatment of Parkinsonism Mainly CNS: Insomnia, confusion, seizure (less frequent with Rimantadine) Arrhythmia 28 Oseltamivir Nature Mechanism of Action Therapeutic use Adverse Effects Sialic acid analog Influenza neuraminidase cleaves terminal sialic acid residues and destroys the receptors recognized by viral hemagglutinin, which are present on the cell surface, which is essential for virus release from infected cells Oseltamivir causes a conformational change in neuraminidase’s active site and inhibits its activity, leading to viral aggregation at the cell surface and reduced virus spread within the respiratory tract Treatment and prevention of influenza A and B virus infections Inhibits amantadine- and rimantadine-resistant influenza A viruses Nausea, abdominal discomfort and less often emesis probably owing to local irritation GI complaints (usually mild) 29 Zanamivir Nature Mechanism of Action Potently Therapeutic use Adverse Effects Sialic acid analog and specifically inhibits the neuraminidases of influenza A and B viruses Treatment and prevention of influenza A and B virus infections Best drug to protect against household transmission Whizzing Bronchospazm 30 31 ANTI-hepetitis VIRUS AGENTS Adefovir Ribavirin Lamivudin Palivizumab 32 Adefovir Nature Acyclic adenosine nucleotide analog Mechanism of Action Cellular enzymes Therapeutic use approved Adverse Effects convert adefovir to the diphosphate, which competitively inhibits viral DNA polymerases and Reverse transcriptases and also serves as a chain terminator of viral DNA synthesis. for treatment of chronic HBV infections This also includs lamivudine-resistant HBV strains Dose related Nephrotoxicity: proteinurea, azotemia, glucosurea 33 Interferons Nature Possess antiviral, immunomodulating, and antiproliferative activities They are synthesized by host cells in response to various inducers and stimulate an antiviral state in cells There are three major classes of human interferons with antiviral activity: α, β and γ Mechanism of Action Interferes Therapeutic use Chronic Adverse Effects Acute with viral replication at multiple steps: penetration, mRNA synthesis, assembly of viral particles, release etc. Also itself synthesize certain Antiviral proteins HBV and HCV infection Broad-spectrum antiviral activity against respiratory viruses Papilloma virus infection in genital warts Genital HSV infections, localized herpes-zoster infection, and CMV infections of renal transplant patients influenza like syndrome: fever, chills, headache, myalgia, arthralgia, nausea, vomiting, and diarrhea (resolved within 12 hrs) Myellosupression 34 35 Lamivudine Nature Cytidine Mechanism of Action Triphosphate Therapeutic use Enhanced Adverse Effects No nucleoside analog form potently inhibits the DNA polymerase / Reverse transcriptase of HBV and DNA polymerase of HIV antiviral activity against hepadnaviruses when combined with adefovir or penciclovir HBV infection in children Administered before and after liver transplantation to suppress recurrent HBV infection marked adverse effects Generally well tolerated 36 Ribavirin Nature Purine Mechanism of Action Inhibits Therapeutic use In Adverse Effects Aerosolized nucleoside analog with a modified base and D-ribose sugar viral mRNA synthesis Interferes with the synthesis of GTP Inhibit viral cell replication in both DNA and RNA viruses combination with parenteral IFN is standard treatment for chronic HCV infection Aerosol is approved in the U.S. for treatment of RSV bronchiolitis and pneumonia in hospitalized children, in bonemarrow transplant and higjly immunocompromised patients form: conjunctival irritation, rash, transient wheezing, and reversible deterioration in pulmonary function Systemic: reversible anemia owing to extravascular hemolysis and bone marrow suppression Teratogenic and Gonadotoxic 37 ANTI-retro VIRUS AGENTS Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NRTI] Non- Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NNRTI] Protease Inhibitor [PI] 38 Anti-Retroviral Agents [2] NTRTI [1] NRTI Zidovudine Tenofovir Stavudine Didanosine Lamivudine Abacavir Zalcitabine [3] NNRTI [4] PI Efavirenz Saquinavir Nevirapine Ritonavir Delavirdine Indinavir Nelfinavir Amprenavir Lopinavir 39 40 41 Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NRTI] 42 Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NRTI] Mechanism of Action: Viral RNA REVERSE TRANSCRIPTASE Proviral DNA Incorporated into host cell chromosome Nucleoside and nucleotide analogs must enter cells and undergo phosphorylation to generate synthetic substrates for the enzyme Reverse Transcriptase Fully phosphorylated analogs block replication of the viral genome by: competitively inhibiting incorporation of native nucleotide terminating elongation of nascent proviral DNA 43 Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NRTI] Mechanism of Action: Viral RNA REVERSE TRANSCRIPTASE Proviral DNA Incorporated into host cell chromosome These compounds inhibit both HIV-1 and HIV-2 and several have broadspectrum activity against other retroviruses; some are also active against HBV and the herpes viruses 44 ZDV: zidovudine [T] d4T: stavudine [T] ddC: dideoxycytidine [C] FTC: emtricitabine [C] 3TC: lamivudine [C] ABC: abacavir [G] ddI: didanosine [A] DF: disoproxil fumarate [A] MP, monophosphate; DP,diphosphate; TP, triphosphate; AMP, adenosine monophosphate; CMP, cytosine monophosphate; dCMP, deoxycytosine monophosphate; IMP, inosine 5′-monophosphate; PRPP, phosphoribosyl pyrophosphate; NDR, nucleoside diphosphate 45 Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NRTI] Adverse Effects: Selective toxicity of these drugs depends on their ability to inhibit the HIV reverse transcriptase without inhibiting host cell DNA polymerases Some are capable of inhibiting human DNA polymerase γ Toxicities due to inhibition of mitochondrial DNA synthesis are frequently observed which are: anemia, granulocytopenia, myopathy, peripheral neuropathy, and pancreatitis 46 Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NRTI] Therapeutic uses: DRUG Zidovudin SPECTRUM HIV-1, HIV-2 USES/COMMENTS FDA-approved for the treatment of adults and children with HIV infection lymphotrophic Preventing mother-to-child transmission viruses (HTLV) I and II Recommended for postexposure prophylaxis in HIV-exposed healthcare workers human T-cell Didenosine HIV-1 HIV-2 HTLV-1 Acid labile, and therefore is administered with an antacid buffer. Food decreases bioavailability 47 Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NRTI] Therapeutic uses: DRUG SPECTRUM Stavudine HIV-1, HIV-2 Zalcitabine Lamivudine USES/COMMENTS Bioavailability is not affected by food A sustained-release formulation that can be given once daily is FDA-approved HIV-1, HIV-2 HBV Food does not affect bioavailability distinctive toxicity: oral ulceration and stomatitis HIV-1, HIV-2 HBV oral bioavailability is >80% and is not affected by food one of the least toxic antiretrovirals Frequently employed in three-drug regimens with other nucleoside analogs, protease 48 inhibitors, and/or NNRTIs Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NRTI] Therapeutic uses: DRUG SPECTRUM Abacavir HIV-1 Tenofovir HIV-1, HIV-2 HBV USES/COMMENTS oral bioavailability is >80% and is not affected by food Most imp A/E: a unique and potentially fatal hypersensitivity Widely used in coformulations with zidovudine or lamivudine Only nucleotide analog currently marketed for HIV infection FDA-approved as a component of combination HIV therapy for adults in triple drug regimen 49 Non-Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NNRTI] 50 Non-Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NNRTI] Mechanism of Action: Noncompetitive inhibitors that bind to a peripheral site on HIV-1 reverse transcriptase. The binding site is a hydrophobic pocket in the p66 subunit of reverse transcriptase, distant from the active site. Occupation of the site by an NNRTI induces a conformational change that greatly reduces the enzyme’s activity. Because the binding site for NNRTIs is strain-specific, the approved agents are active against HIV-1 but not HIV-2 or other retroviruses. They have no activity against host cell DNA polymerases. NNRTIs do not require intracellular phosphorylation for activity. Adverse Effect: Rash and severe hepetitis 51 Non-Nucleoside and Nucleotide Reverse Transcriptase Inhibitors [NNRTI] Therapeutic uses: DRUG Nevirapine USES/COMMENTS FDA-approved for the treatment of HIV-1 infection in adults and children Used to prevent mother-to-child transmission of HIV-1 Delavirdine Least used of the NNRTIs Avoided with potent enzyme inducers Efavirenz Widely used because of its convenience, effectiveness, and long-term tolerability Predominant adverse effects of CNS: dizziness, impaired concentration, dysphoria Teratogenic 52 Protease Inhibitors [PI]: Mechanism of Action: Competitively inhibit the action of the viral enzyme Aspartyl protease This protease is a homodimer consisting of two 99-amino-acid monomers; each monomer contains an Aspartate residue that is essential for catalysis. The preferred cleavage site for this enzyme is the N-terminal side of Proline residues, especially between Phenylalanine and Proline Human aspartyl proteases contain only one polypeptide chain and are not significantly inhibited by HIV protease inhibitors 53 Protease Inhibitors [PI]: Mechanism of Action: These drugs bind reversibly to the active site of the protease and prevent proteolytic cleavage of HIV gag and pol proteins into essential structural and enzymatic components of the virus. This prevents themetamorphosis of HIV virus particles into their mature infectious form 54 Protease Inhibitors [PI]: Adverse Effects: Most commonly: nausea and vomiting, anorexia, diarrhea Unique: lipodystophy, a metabolic syndrome characterized by insulin resistance and dyslipidemia 55 Protease Inhibitors [PI]: Saquinavir Inhibits HIV-1 and HIV-2 replication. Of all of the HIV protease inhibitors, saquinavir inhibits CYP3A4 least potently. Administered in combination with ritonavir Ritonavir Active against HIV-1 and HIV-2. Extremely potent inhibitor of CYP3A4 and is frequently combined with most of the PI. Better tolerated 56 Protease Inhibitors [PI]: Indinavir Food adversely affects indinavir bioavailability Unique adverse effects of indinavir are crystalluria and nephrolithiasis Nelfinavir Active against HIV-1 and HIV-2 Very sensitive to food effects Used in HIV-infected patients with significant hepatic dysfunction Lopinavir Inhibits both HIV-1 and HIV-2. Only administered in combination with ritonavir Should be taken with food because oral bioavailability is increased 57 by 50% with a high fat meal. Entry Inhibitors [PI]: Enfuvirtide Mechanism of action: Prevents formation of a six-helix bundle critical for membrane fusion and viral entry into the host cell Inhibits infection of CD4 cells by free virus particles, as well as cell-to-cell transmission of HIV-1 Adverse effects: Injection site reactions including: pain, erythema, nodules or cysts Therapeutic Use: FDA-approved for use only in treatment of adults resistant to other applied HIV-1antiretroviral therapy 58 References: 1. 2. 3. Brunton L., Parker K., Blumenthal D., Buxton L., Goodman and Gillman’s Manual of Pharmacology and Therapeutics; New York: McGraw Hill; 2008; P. 814-912. Harrison TR.,Kasper DL., Braunwald E., Fauci AS., Hauser SL., Longo DL. et al.; Harrison’s Principles of Internal Medicine. New York: McGraw Hill; 2005; p.1035-1040. Rang HP., Dale MM., Ritter JM., Flower RJ., Rang and Dale’s Pharmacology, China: Elsevier publishers; 2007; p. 679-683. 59 60