* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download supports decisions
Survey
Document related concepts
Transcript
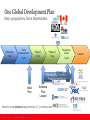
Regulatory and Reimbursement Harmonization An Industry Perspective Adrian Griffin | April 2016 One Global Development Plan Many geographies, More Stakeholders Discovery 3 yrs Early Development 2 yrs Phase II 2 yrs Phase III 3 yrs Regulatory review 1-2yrs Launch Planning the Evidence Predicting the future Initial Plan Evidence Plan ‘Locked’ Clinical Use Need to know evidence requirements 4-7 yrs before launch 2 Need for Regulatory and HTA Agencies to be Aligned Focus of HTAi Policy Forum, 2011 International Journal of Technology Assessment in Health Care, 27:3 (2011), Page 1 of 8. 3 Different Focus, Different Decisions Both driving for better outcomes for patients Regulation HTA Coverage Give market authorisation Support for clinical and coverage decisions Decide on coverage or reimbursement Decision Do the clinical benefits for patients outweigh the risks? [HTA supports decisions, often taking into account clinical, financial & other dimensions] Are the expected health benefits useful and affordable? Evidence Effectiveness, cost Efficacy and safety from effectiveness and trials; post launch opportunity costs from surveillance trials, other studies and modelling As for HTA; conditional coverage may be used to improve evidence base for re-appraisal Role 4 Regulatory-HTA Interactions have been Increasing Timing • Pre-competitive Type of Interaction • Disease Specific – Evidence Guidance Documents • Green Park Collaborative • During Development – Pre-Phase II / III • Pre-Market / launch • Early Scientific Advice – Product specific advice – HTA, Multi-HTA, Parallel Reg-HTA • Parallel reviews – Regulatory and reimbursement • Canada, Australia, US 5 New Challenges for Stakeholders • Regulator, patient, clinicians call for ‘early access’ – FDA: Breakthrough therapies – EMA: PRIME • Regulators discussing longitudinal approach to approval – “Adaptive Licensing” Now Medicines Adaptive Pathways to Patients (MAPPs) • How will HTA & Reimbursement adapt? – Existing approach to value assessment needs to evolve 6 The Challenges are here now Crossover bias Ibrutinib versus Ofatumumab in previously treated CLL: Byrd et al, NEJM, July 2014 Ofatumumab as single agent CD20 immunotherapy in fludarabine-refractory CLL: Wierda et al, JCO, April 2010 7 Ongoing Initiatives 8 Ongoing Initiatives (2) To show how Real World Evidence (RWE) can be adopted in to medicine development & decision making, and provide the tools to achieve it An enabling platform to coordinate MAPPs activities. Supporting evidence generation, designing the MAPPs pathway, decision-making & sustainability https://www.imi-getreal.eu/ http://adaptsmart.eu/ Both are multi-stakeholder, including regulators, HTA agencies, patient organisations, academics and industry 9 Countries can find ways to deliver access, whilst managing their uncertainty Scheme type Rebate for early non-responders Range of Managed Entry Agreement Archetypes in use in Europe, for One Drug in one Disease Capped treatment duration Treatment duration linked rebate Response scheme Initiation period costs Population level cap 10 Challenges • Regulators are Decision-Makers – Able to change their decision-making paradigm • HTA-agencies provide information & recommendations – Third parties then make Reimbursement decisions • What flexibility do HTA-agencies have to navigate the reimbursement access model? – Will a new model be required in the new regulatory environment • Industry needs to address Regulators, HTAs, Clinicians and Patients 11