* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PowerPoint - Name Hydrocarbons
Survey
Document related concepts
Transcript
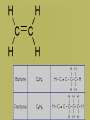
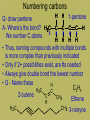
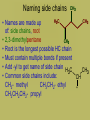
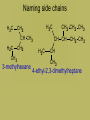
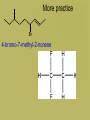
Organic Chemistry • Carbon is the basis of organic chemistry • Carbon has the ability to make 4 covalent bonds. • Carbon can repeatedly make covalent bonds to itself to make rings and chains. • Carbon can make single, double or triple bonds to itself and other elements. • Aliphatic compounds are hydrocarbons which form a chain which may be straight or branched, not rings. • When naming organic compounds, every compound should have its own unique name. The # of carbon atoms present must be indicated. Each name has a prefix which states the # of carbon atoms. Alkanes • Simplest hydrocarbons, they contain only carbon to carbon bonds (single bonds). • The suffix –ane is used to show an Alkane (only single bonds). To name Alkane Nomenclature • 1. Find the longest continuous carbon chain • 2. Use prefixes (table P) to indicate # of carbon atomsi n longest chain. • 3. Use suffix “ane” to indicate single bonds Alkenes & Alkynes • Hydrocarbons which contain one double bond between carbon atoms. • They use the suffix ene in naming • Alkynes are hydrocarbons which contain one triple bond • They use the suffix yne in naming Unsaturated hydrocarbons • Since the molecule has a double or triple bond, there are less H’s on the chain. His looks like they are missing H’s, we classify them as unsaturated. • Alkanes are saturated hydrocarbons. They have the max # of H’s on the chain – others are not. Isomer • When molecules have the same formula but different structre. • Different substances, have different properties from each other. • To give each isomer a name you need to use a # to indicate which C atom the double bond starts (lowest possible number is given to carbon closest to double bond) C4H8 These molecules are different because their structures are different (double bond is in different places) Numbering carbons Q- draw pentene A- Where’s the bond? H3C 5 1 We number C atoms H H 1-pentene C C C C H H H H 4 2 3 2 4 1 5 H • Thus, naming compounds with multiple bonds is more complex than previously indicated • Only if 2+ possibilities exist, are #s needed • Always give double bond the lowest number • Q - Name these H C 2H 4 H 3C C 2-butene C CH3 Ethene H H 3C CH3 3-nonyne Cyclic structures H H H H C C C H • Cyclic structures are circular C C • Have “cyclo” in name H H H H • Benzene is not a cyclic structure • cyclopentane H CH2 H2C CH2 Benzene Ring C6H6 • Contains 6 carbons and 3 double bonds Branched Alkanes • These are saturated hydrocarbons which have carbon atoms which hang from the main chain. They are not a straight chain. • Functional Group- is a group of atoms which hang off the main chain • Methyl Group- only has a single carbon in the branch ex. CH3 • Ethyl group has two carbons in a branch • ex: CH3CH2 • Propyl group has 3 carbons branched • Ex:CH3CH2CH2 • If there are more than one functional group you must write prefix, di, tri, tetra Naming side chains CH3 CH33 H3C • Names are made up of: side chains, root • 2,3-dimethylpentane CH3 • Root is the longest possible HC chain • Must contain multiple bonds if present • Add -yl to get name of side chain H C CH3 3 • Common side chains include: CH CH3- methyl CH3CH2- ethyl * CH3CH2CH2- propyl Naming side chains CH2 ethyl CH2 CH3 1 CH3 CH2 C 2 5 CH2 C 3 4 CH3 6 CH3 methyl methyl Rule 8,9: group similar branches 2-ethyl-4,4-dimethyl-1-hexene Naming side chains H3C H 3C CH2 H 2C CH CH3 CH2 CH3 3-methylhexane CH2 CH2 CH3 CH CH CH2 CH3 H3C CH CH3 4-ethyl-2,3-dimethylheptane Naming side chains Name the structures below CH3 CH H 3C CH2 CH CH3 H 2C H3C H3C 3-ethyl-2-methylpentane CH3 CH3 3-ethyl-1,5,5trimethylcyclohexene CH3 IUPAC Rules for Halocarbon Nomenclature • 1. Find and name the longest continuous carbon chain. • 2. Identify and name groups attached to this chain. Using the prefixes for each halogen. • 3. Number the chain consecutively, starting at the end nearest a substituent group. • 4. Assemble the name, listing groups in alphabetical order. • 6. The prefixes di, tri, tetra are used to designate several groups of the same kind, are not considered when alphabetizing. • Cl- chloro, F- Fluoro, Br- Bromo More practice Br 4-bromo-7-methyl-2-nonene Alcohols • An Alcohol is a substance where one or more carbons within a hydrocarbon are replaced with a –OH • -OH is called a hydroxyl group • Only one –OH can be attached to one carbon. • CRT: R—OH • Replace ending of name with -ol Name this Alcohol Ether • An organic compound that contains an ether group • Ether group- an oxygen atom connected to 2 hydrocarbon branches • CRT: R-O-R • IUPAC says: name each branch connected to O independently from each other • -change ending to –yl of each group than add ether to the end of the name. Name this Ether! Aldehydes • Compounds containing the functional group called the carbonyl group. • Carbonyl group is a carbon double bonded to an oxygen at their the start or end of a chain. • CRT: Drop the –e ending from the hydrocarbon and change it to -al • Name this Aldehyde! Ketones • Contain a carbonyl group within the middle of a carbon chain. • CRT: • In order to name ketones, you drop the –e ending from the hydrocarbon name and add an –one. • The location of the =O on the chain must be indicated with a number. Name that Ketone! Practice- Name each: Organic Acids • Carboxylic acids contain a -COOH group at the first (last) Carbon. • Usually written R-COOH or R-CO2H • Very weak acids that do not break up completely in water (dissociate). • IUPAC naming : replace final –e with • –oic acid Name this Organic Acid! Esters • Esters are derived from carboxylic acids. • Any carboxylic acid contains –COOH group. • In an Ester, a hydrogen (not on the first carbon) is replaced with a –COO symbolized as R-COO-R • Esters smell nice, frangrances of many flowers, used in perfume, responsible for tastes of ripened ruits, Beeswax. • Esters look like 2 they are in 2 parts. • First name the alkyl (R) group (part hanging off of acid part) • Than name the group which contains the double bond (R-COO-R) • For this group, replace the oic acid as used previously with –oate. Name that Ester! Amines • Think of Ammonia NH3 • In amines, the hydrogen atoms in the ammonia are replaced one at a time by hydrocarbon groups. • There are different types of amines based on how many hydrogen atoms are replaced. • In primary amines, that means 1 hydrogen has been replaced RNH2 where R is an alkyl group • In secondary amines, 2 hydrogens are replaced. • In tertiary amines, all of the hydrogens in an ammonia molecule have been replaced. How to name: • 1. name each branch of the hydrocarbon chain attached to the Nitrogen and end with amine. • 2. –NH2 is an amino group, so you can name the hydrocarbon as usual but begin the name with amino • 3. Name the longest hydrocarbon chain and replace the –e in –ane with amine. What are the 3 different names of this amine? • 1. 2-Propyl Amine • 2. 2- Amino Propane • 3. 2-Propanamine Amides • Amides are derived from carboxylic acids meaning the –COOH group • In an amide the OH part of that group is replaced by an –NH2 group • R-CONH2 • IUPAC naming: replace the –e at the end with an -amide Example Name this amide! • What would • Ethyl ethanamide • Look like?