* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Variation of the dielectric constant in alternating fields We know that
Aharonov–Bohm effect wikipedia , lookup
Speed of gravity wikipedia , lookup
Gibbs free energy wikipedia , lookup
Field (physics) wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Time in physics wikipedia , lookup
Casimir effect wikipedia , lookup
Superconductivity wikipedia , lookup
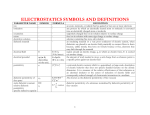
Variation of the dielectric constant in alternating fields We know that a dielectric becomes polarised in an electric field. Now imagine switching the direction of the field. The direction of the polarisation will also switch in order to align with the new field. This cannot occur instantaneously some time is needed for the movement of charges or rotation of dipoles. If the field is switched, there is a characteristic time that the orientational polarisation or average dipole orientation takes to adjust, called the relaxation time. Typical relaxation times are s. Therefore, if the electric field switches direction at a frequency higher than Hz, the dipole orientation cannot keep up with the alternating field, the polarisation direction is unable to remain aligned with the field, and this polarisation mechanism ceases to contribute to the polarisation of the dielectric. In an alternating electric field both the ionic and the electronic polarisation mechanisms can be thought of as driven damped harmonic oscillators like a mass on a spring, and the frequency dependence is governed by resonance phenomena. This leads to peaks in a plot of dielectric constant versus frequency, at the resonance frequencies of the ionic and electronic polarisation modes. A dip appears at frequencies just above each resonance peak, which is a general phenomenon of all damped resonance responses, corresponding to the response of the system being out of phase with the driving force we shall not go into the mathematical proof of this here. In this case, in the areas of the dips, the polarisation lags behind the field. At higher frequencies the movement of charge cannot keep up with the alternating field, and the polarisation mechanism ceases to contribute to the polarisation of the dielectric. As frequency increases, the materials net polarisation drops as each polarisation mechanism ceases to contribute, and hence its dielectric constant drops. The animation below illustrates these effects. The dielectric constant The dielectric constant of a material provides a measure of its effect on a capacitor. It is the ratio of the capacitance of a capacitor containing the dielectric to that of an identical but empty capacitor. An alternative definition of the dielectric constant relates to the permittivity of the material. Permittivity is a quantity that describes the effect of a material on an electric field the higher the permittivity, the more the material tends to reduce any field set up in it. Since the dielectric material reduces the field by becoming polarised, an entirely equivalent definition is that the permittivity expresses the ability of a material to polarise in response to an applied field. The dielectric constant sometimes called the relative permittivity is the ratio of the permittivity of the dielectric to the permittivity of a vacuum, so the greater the polarisation developed by a material in an applied field of given strength, the greater the dielectric constant will be. There is no standard symbol for the dielectric constant you may see it referred to as , , or r. In this TLP shall be used to avoid confusion with the absolute permittivity, which may also be given the symbol . Effect of structure on the dielectric constant We have already seen that the more available polarisation mechanisms a material possesses, the larger its dielectric constant will be. For example, materials with permanent dipoles have larger dielectric constants than similar, nonpolar materials. In addition, the more easily the various polarisation mechanisms can act, the larger the dielectric constant will be. For example, among polymers, the more mobile the chains are i.e. the lower the degree of crystallinity the higher the dielectric constant will be. For polar structures, the magnitude of the dipole also affects the magnitude of polarisation achievable, and hence the dielectric constant. Crystals with noncentrosymmetric structures such as barium titanatehave especially large spontaneous polarisations and so correspondingly large dielectric constants. Conversely, a polar gas tends to have smaller dipoles, and its low density also means there is less to polarise, therefore polar gases have lower dielectric constants than polar solids or liquids. The density argument also applies for nonpolar gases when compared with nonpolar solids or liquids. Effect of temperature on the dielectric constant For materials that possess permanent dipoles, there is a significant variation of the dielectric constant with temperature. This is due to the effect of heat on orientational polarisation. e This animation requires Adobe Flash Player and later, which can be downloaded here. However, this does not mean that the dielectric constant will increase continually as temperature is lowered. There are several discontinuities in the dielectric constant as temperature changes. First of all, the dielectric constant will change suddenly at phase boundaries. This is because the structure changes in a phase change and, as we have seen above, the dielectric constant is strongly dependent on the structure. Whether will increase or decrease at a given phase change depends on the exact two phases involved. There is also a sharp decrease in at a temperature some distance below the freezing point. Let us now examine the reason for this. In a crystalline solid, there are only certain orientations permitted by the lattice. To switch between these different orientations, a molecule must overcome a certain energy barrier E. When an electric field is applied, the potential energy of orientations aligned with the field is lowered while the energy of orientations aligned against the field is raised. This means that less energy is required to switch to orientations aligned with the field, and more energy required to switch to orientations aligned against the field. Therefore over time molecules will become aligned with the field. However, they must still overcome an energy barrier in order to do this. If a molecule possesses an energy less than the height of any energy barrier, it cannot cross the energy barrier therefore cannot change its orientation. Hence the orientational mode becomes frozen out and can no longer contribute to overall polarisation, leading to a drop in the dielectric constant. These effects are summarised in the graph below. Loss in dielectrics An efficient dielectric supports a varying charge with minimal dissipation of energy in the form of heat. There are two main forms of loss that may dissipate energy within a dielectric. In conduction loss, a flow of charge through the material causes energy dissipation. Dielectric loss is the dissipation of energy through the movement of charges in an alternating electromagnetic field as polarisation switches direction. Dielectric loss is especially high around the relaxation or resonance frequencies of the polarisation mechanisms as the polarisation lags behind the applied field, causing an interaction between the field and the dielectrics polarisation that results in heating. This is illustrated by the diagram below recall that the dielectric constant drops as each polarisation mechanism becomes unable to keep up with the switching electric field. Dielectric loss tends to be higher in materials with higher dielectric constants. This is the downside of using these materials in practical applications. Dielectric loss is utilised to heat food in a microwave oven the frequency of the microwaves used is close to the relaxation frequency of the orientational polarisation mechanism in water, meaning that any water present absorbs a lot of energy that is then dissipated as heat. The exact frequency used is slightly away from the frequency at which maximum dielectric loss occurs in water to ensure that the microwaves are not all absorbed by the first layer of water they encounter, therefore allowing more even heating of the food. Dielectric breakdown At high electric fields, a material that is normally an electrical insulator may begin to conduct electricity i.e. it ceases to act as a dielectric. This phenomenon is known as dielectric breakdown. The mechanism behind dielectric breakdown can best be understood using band theory. A detailed explanation of this can be found in the TLP on semiconductors although not all of this is relevant to the content of this TLP, therefore the aspects of band theory needed to understand dielectric breakdown are presented here. Applications of dielectrics A major use of dielectrics is in fabricating capacitors. These have many uses including storage of energy in the electric field between the plates, filtering out noise from signals as part of a resonant circuit, and supplying a burst of power to another component. The TLP on ferroelectrics shows how the last of these functions is utilised in a camera flash system. The larger the dielectric constant, the more charge the capacitor can store in a given field, therefore ceramics with noncentrosymmetric structures, such as the titanates of group metals, are commonly used. In practice, the material in a capacitor is in fact often a mixture of several such ceramics. This is due to the variation of the dielectric constant with temperature discussed earlier. It is generally desirable for the capacitance to be relatively independent of temperature therefore modern capacitors combine several materials with different temperature dependences, resulting in a capacitance that shows only small, approximately linear temperaturerelated variations. Of course in some cases a low dielectric loss is more important than a high capacitance, and therefore materials with lower values of and correspondingly lower dielectric losses may be used for these situations. Some applications of dielectrics rely on their electrically insulating properties rather than ability to store charge, so high electrical resistivity and low dielectric loss are the most desirable properties here. The most obvious of these uses is insulation for wires, cables etc., but there are also applications in sensor devices. For example, it is possible to make a type of strain gauge by evaporating a small amount of metal onto the surface of a thin sheet of dielectric material. Electrons may travel across the metal by normal conduction, and through the intervening dielectric material by a phenomenon known as quantum tunnelling. A mathematical treatment of this phenomenon is outside the scope of this TLP simply note that it allows particles to travel between two permitted regions that are separated by a forbidden region and that the extent to which tunnelling occurs decreases sharply as distance between the permitted regions increases. In this case the permitted regions are the solidified metal droplets, and the forbidden region is the highresistance dielectric material. If the dielectric material is strained, it will bow causing the distances between the metal islands to change. This has a large impact on the extent to which electrons can tunnel between the islands, and thus a large change in current is observed. Therefore the above device makes an effective strain gauge. Summary Dielectrics are electrical insulators that support charge. The properties of dielectrics are due to polarisation. There are three main mechanisms by which polarisation arises on the microscopic scale permanent dipoles. electronic distortion of the electron cloud in an orientational rotation of atom, ionic movement of ions and A capacitor is a device that stores charge, usually with the aid of a dielectric material. Its polarise. It can be capacitance is defined by Q C V The dielectric constant indicates the ability of the dielectric to defined as the ratio of the dielectrics permittivity to the permittivity of a vacuum. Each of the polarisation mechanisms has a characteristic relaxation or resonance drop. frequency. In an alternating field, at each of these dielectric constant will sharply materials dependent frequencies, the The dielectric constant is also affected by structure, as this affects the ability of the material to polarise. Polar dielectrics show a decrease in the dielectric constant as temperature increases. Dielectric loss is the absorption of energy by movement of charges in an alternating field, and is particularly high around the relaxation and polarisation mechanisms. resonance frequencies of the Sufficiently high electric fields can cause a material to undergo dielectric breakdown and become conducting.