* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download MDMA - Multidisciplinary Association for Psychedelic Studies
Survey
Document related concepts
Transcript
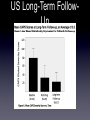
Developing Psychedelics into Prescription Medicines: MDMA-Assisted Psychotherapy for Posttraumatic Stress Disorder By Rick Doblin, PhD, Executive Director Multidisciplinary Association for Psychedelic Studies Entheogenesis Australias Replacing Fears... With Facts find of a drug lab they had raided and taken a photo of.” -Dr. David E. Nichols, Ph.D. • “I love the way the way they always throw the battery acid and drain cleaner into the story, as if prescription drugs were not manufactured using the exact same reagents, which are simply dilute sulfuric acid and sodium hydroxide!” -Dr. Nicholas Vito Cozzi, Ph.D. Brain SPECT ScanMTV/Oprah Holes in the Brain from MDMA? (Ecstasy. Create Fear.) Leshner Testimony to Senate Subcommittee on Government Affairs 7/30/01 Bottle from experiement of MDMA Harvard MDMA Cancer - ask John Documentary Clip • • • • • • • Outline Why MDMA? Why PTSD? Questions for Phase II trials US Pilot study & follow-up: Design & Results International series of Phase II studies US MDMA/PTSD war veterans study Australian MDMA/PTSD study? Why MDMA? • • • • Reduces fear response to perceived emotional threat (reduced activity in amygdala; Gamma, A., et al., 2002) Increases trust and empathy (oxytocin, prolactin, similar to post-orgasmic state; Passie et al., 2005) Gentle but profound; easier to integrate Therapists/psychiatrists more willing to self-experiment than classic psychedelics Why MDMA? • • • Highly demonized: 3500+ papers in Medline about risks/mechanisms of action Estimated cost $300 million worth of studies in the public domain MAPS spent $150,000 to read and summarize all published scientific peerreviewed literature on MDMA • • • • Why PTSD? Current treatment approaches fail to provide relief in a substantial number of patients Internationally accepted outcome measure (CAPS) Therapeutic change captured by outcome measure (not the case with psychedelic therapy for end-of-life anxiety & depression) Highly sympathetic patient population (Veterans, survivors of sexual assault, abuse, accidents, and trauma) Why PTSD? PTSD A fear-based disorder MDMA Treatment for fear $300 million of existing research Millions of users, rare side effects known = Top priority combination of psychedelic drug and clinical condition Phase II Methodological • DevelopmentIssues of our therapeutic model • Training of therapists • Cause of PTSD related to treatment method? • Successful double-blind design • Variance and magnitude of treatment effect • Cultural differences Development of our Therapeutic Model Can be viewed at www.maps.org Therapists • Training 5 day training program mostly watching videos and reviewing treatment manual and adherence criteria • Psychological effects of MDMA administration in normal volunteers training to conduct MDMA-assisted psychotherapy research • Review video tapes of first two therapy sessions by raters scoring adherence criteria with feedback from Mithoefers Cause of PTSD Related to Treatment Method? •Sexual assault vs. war-related trauma? Successful Double-Blinding • Inactive placebo • Active placebo • Dose response Treatment Effect (Variance & Magnitude) • Data from US versus Swiss • get Swiss data from Ilsa Cultural Differences US MDMA/PTSD Pilot Study Design • • • • • Treatment resistant/pharmacotherapy & psychotherapy Average duration PTSD = 19 years Inactive placebo versus 125mg + 62.5mg Combination: drug & non-drug psychotherapy 2-3 MDMA sessions with 2 month & longterm follow-up Treatment Process Non-Drug Integrative Therapy Sessions Non-Drug Integrative Therapy Sessions 1 1 1 2 2 2 90- Min Non-Drug 90-Minute Non-Drug Integrative Intro Therapy Sessions Therapy Sessions Screenin g/Baselin e 1 2 3 Exp Sessio n MDMA or Placebo • • • 3 Exp Sessio n MDMA or Placebo 3 Exp Sessio n 3 O u t c o m e F o l l o w U p MDMA or Placebo In addition, therapists have up to a 30-minute phone session with participant for 7 days following each experimental session. Outcome Assesment = 2 months after last experimental session Follow-up Assessment = 12 months after last experimental session get from ilsa orMM this yellow colum should be 25% US Long-Term FollowUp Swiss MDMA/PTSD • Improvements not observed in active placebo group 25mg + 12.5mg BUT did produce some blinding • MDMA group benefited, but not as much as in US MDMA/PTSD study • No Serious Adverse Events (no evidence of harm) • Long-term follow-up to be completed Jan 2011; paper submitted to journal by April 2011 • graph of Swiss data Swiss • Physiological correlates of PTSD study conducted by Dr Franz X. Vollenweider, M.D. @ University of Zurich in all 12 participants • EEG • Startle reflex • Heart rate variability (HRV) • Results will be published as a separate paper Israel • 5 patients • No Serious Adverse Events (no evidence of harm) • No efficacy Current & Upcoming Studies • Canada • Israel • Jordan • US MDMA/PTSD War Veterans Canada • Can we replicate US results in a similar cultural context with similarly trained therapists? • N = 12 • 125mg + 62.5mg versus 25mg + 12.5mg • 3 sessions • Waiting on import permit Israel • Different cultural context • 2 sessions instead of 3 • N = 10 • 125 + 62.5 versus 25 + 12.5 • Traditional psychiatrists paired with psychologists experienced with PTSD or MDMA Jordan • Different cultural context • How do we teach our method? • N = 12 Iraqi refugees in Jordan • 3 Sessions • 125 + 62.5 experimental dose • Using a 40mg +20mg low dose- another attempt to work on double-blind • Arabic caps on next slide Arabic CAPS US: MDMA/PTSD Study in War Veterans • To see if cause of PTSD requires different method • Investigate double-blind: 3 dose groups: Low (30 mg + 15mg), medium (75 mg + 37.5) and full dose (125 mg + 62.5) • Higher risk populations (controlled hypertension and Hepatitis C) • Potential design for Phase III studies Timeline • 2-3 years to FDA End of Phase II Mtg • 3-5 years for all Phase 3 studies • 1-2 years for FDA review Zulfi July 21, 2010 Text Australian MDMA/PTSD • 25k matching grants for Australia MDMA/PTSD • + Protocol devel and approval assistance • + therapist training • + clinical research oversight • email from someone who contacted rick from Aus- Vets in Aus who wanted to come participate in US study