* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Representation of gas-condensate wells in reservoir simulations
Survey
Document related concepts
Transcript
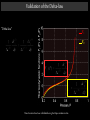
Laboratoire d'Énergétique et de Mécanique Théorique et Appliquée Ecole Nationale Supérieure de Géologie Institut National Polytechnique de Lorraine STREAMLINE SPLITTING THE THERMO- AND HYDRODYNAMICS IN COMPOSITIONAL FLOW THROUGH POROUS MEDIA APPLICATION TO H2-WATER IN RADIOACTIVE WASTE DEPOSITS S. OLADYSHKIN, M. PANFILOV 1 Sommaire PresentatIon Introduction Flow Model Limit compositional model Streamline HT-splitting Validation to the limit thermodynamic model 2 Introduction Physical description 3 Hydrogen generation in a radioactive waste deposit Gas generation: H2 + CO2 + N2 + O2 + … Storage pressure growth : Corrosion in storage tank - Initial : 100 bar - Increased by H2 : 300 bar Water Monitoring problem : H2 transport through porous media 4 accompanied with radionuclides Fluid structure Phases : Components : Gas Liquid H2 CO2 N2 O2 H20 … 2 phases Gas Liquid 5 Similar phenomena in an underground H2 storage Well Hydrogen storage Well GAS and LIQUID H20 + H2 + CO2 + CH4 + … 6 Phase behaviour Critical point L Initial state G L+G 7 Flow Model 8 Compositional model 2 phases (gas & liquid) N chemical components Mass balance for each chemical component k : Momentum balance for each phase (the Darcy law) Phase equilibrium : ( = the chemical potential) or Phase state : Closure relationships: or 9 Limit contrast compositional model 10 Canonical dimensionless form of the compositional model gas flow liquid flow transport of basic chemical components 11 Mathematical type of the system Parabolic equation Hyperbolic equation 12 Characteristic parameters of a gas-liquid system gas flow liquid flow transport of basic chemical components 13 Characteristic parameters of the system Perturbation parameter: Perturbation propagation time Reservoir depletion time Parameter of relative phase mobility: 14 Limit behaviour gas flow liquid flow transport of basic chemical components Semi-stationarity : p and C(k) are steady-state, while s is non stationary 15 Streamline HT-splitting 16 Integration of the transport subsystem Asymptotic contrast compositional model : gas flow liquid flow transport of basic chemical components This subsystem can be integrated along streamlines : A differential thermodynamic system 17 HT-splitting Hydrodynamic subsystem (limit hydrodynamic model): Thermodynamic subsystem (limit thermodynamic model): 18 Split Thermodynamic Model variation of the total composition in an open system Properties The thermodynamic independent system is monovariant: all the thermodynamic variables depend on pressure only The new thermodynamic model is valid along streamlines 19 Thermodynamic “Delta-law” Due to the monovariance, the thermodynalmic differential equations may be simplified to a “Delta-law”: “Delta-law” 20 Interpretation of the delta-law Individual gas volume Individual condensate volume 21 Split Hydrodynamic Model gas flow liquid flow 22 Validation to the limit thermodynamic model 23 Validation of the Delta-law F1 F2 These functions have been calculated using Eclipse simulation data for a dynamic system 24 Flow simulation: Fluid properties Phase plot P Initial conditions: P0 = 315 bar T = 363 K Fluid composition CH4 H2 C10H22 T 25 Flow simulation: Flow problem Well 26 Validation of the Delta-law “Delta-law” F1 F2 These functions have been calculated using the Eclipse simulation data 27 Validation of the total limit thermodynamic model Composition variation in an open thermodynamic system Liquid mole fractions Gas mole fractions Compositional Model (Eclipse) - points; Limit thermodynamic model - solid curves 28 Finita 29