* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Early Embryonic Mesoderm Development
Survey
Document related concepts
Transcript
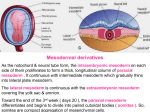
P436643-V1-24 12/24/04 5:56 PM Page 273 24 Early Embryonic Mesoderm Development Virginia E. Papaioannou Introduction Mesoderm—from the Greek µεσο-ζ ′ (middle) + δερµα ′ (skin). This simple descriptive name belies a multifaceted role for the middle of the three embryonic germ layers. It is the youngest layer, in evolutionary terms, and is a hallmark of the development of all complex metazoans. The mesoderm layer provided the solution to more sophisticated functions than the simple protective outer ectoderm and the absorptive inner endoderm. As organisms became larger and more complex, the mesoderm assumed functions of support, movement, circulation, and reproduction, working closely with internalized, ectoderm-derived neural and neural crest tissue as well as providing a supporting role and providing for intricate elaborations of the protective and absorptive functions of the ectoderm and endoderm. With increasingly complex modes of reproduction, all three germ layers were called into play to form novel tissues for the adaptation to different modes of oviparity, ovoviviparity, and eventually viviparity. The repertoire of tissues formed by the mesoderm is complex and varied, with many precursor cell and stem cell populations developing during embryonic life and some persisting into adulthood. The mesoderm plays a role throughout the development of the mammalian embryo, beginning from the initiation of gastrulation, the process whereby the three embryonic germ layers are formed. Here is a brief developmental history of that remarkable tissue in mammals, using the mouse as the prime example. The mammalian embryo begins implantation into the uterus at the late blastocyst stage, when it consists of an outer layer of trophectoderm and a bilaminar inner cell mass (ICM) comprising the epiblast and the primitive endoderm (also called the hypoblast) (Fig. 24–1A). The trophectoderm is a specialized ectoderm layer that mediates contact between the embryo and the uterus and forms a major part of the placenta; it does not, however, make any cellular contribution to the body of the fetus. Similarly, the primitive endoderm layer of the ICM forms the endoderm layer of the visceral and parietal yolk sacs but does not contribute to the gut endoderm of the fetus. It is the epiblast that is the origin of the entire body of the fetus, including ectoderm-, endoderm-, and mesodermderived structures. These three primary layers of cells are called the definitive germ layers and arise through the process Handbook of Stem Cells Volume 1 Copyright © 2004 by Elsevier Inc. All rights of reproduction in any form reserved. of gastrulation, a morphogenetic process that occurs within the epiblast in a specialized structure called the primitive streak. Primitive Streak as the Origin of Mesoderm The first mesoderm to appear in the mammalian embryo is destined to be extraembryonic in nature. One can think of this precocious appearance of mesoderm in the extraembryonic region as an adaptation to viviparity, where a functioning mesoderm-derived circulatory system is an early requirement of successful intrauterine development. In the mouse, this extraembryonic mesoderm arises from the primitive streak, although in humans it appears prior to the formation of a primitive streak. In human embryos, it is said to arise from yolk sac cells,1 which consist at this time of cytotrophoblast and hypoblast; however, the actual origin of this tissue in humans is unknown because of the impossibility of carrying out experimental analysis. Whatever its origin, it comes to have a similar relationship to the other germ layers in the fetal membranes as it does in other mammals, including the mouse. Extraembryonic mesoderm forms and lines the extraembryonic coelom or exocoelomic cavity, providing the mesoderm component of the amnion, chorion, yolk sac, and allantois. The primitive streak of the mouse first appears about 5.5 days postcoitus (dpc) in the epiblast at the junction between the embryonic and extraembryonic areas of the mouse egg cylinder (Fig. 24–1B), marking the posterior pole of the future embryonic body. The streak is an area in which cells of the epiblast undergo an epithelial to mesenchymal transition and commence ingressing, eventually taking up a position and spreading between the visceral endoderm and the epiblast in the embryonic region and between the visceral endoderm and the extraembryonic ectoderm in the extraembryonic region (Fig. 24–1C). The primitive streak gradually elongates with the growth of the embryo until at its maximum length, it extends from the embryonic–extraembryonic boundary to the most distal tip of the egg cylinder. The anterior end of the primitive streak is a specialized area known as the node (or Hensen’s node), which appears as a bilaminar depression at the distal tip of the egg cylinder (Fig. 24–1D). The node has organizing capabilities in that it can induce a secondary neural axis in heterotopic grafting experiments.2 The streak regresses posteriorly toward the latter part of gastrulation, becoming relatively shorter until at 9.5 dpc it is replaced by the tail bud as the source of new mesoderm in the most posterior part of the embryo. 273 P436643-V1-24 30/07/04 1:01 AM Page 274 Virginia E. Papaioannou Epiblast A. Primitive endoderm (hypoblast) Trophectoderm B. Extraembryonic ectoderm Visceral endoderm Primitive streak Epiblast C. Extraembryonic coelom Primitive streak Pr o a m n i o t i c c avi t y D. C h or i o n Yo l k s a c E. A l l a nto i s Amnion Head fold Pe r ic ar dial c av it y P r i m i t i ve s t r e a k Node Hear t Node Cephalic m es oder m Somites Figure 24–1. Diagrammatic representation of midsagittal sections of mouse embryos from 4.5 to 8.5 dpc. (A) A 4.5-dpc blastocyst, just prior to implantation. (B) Early gastrula at 5.5 dpc, with the primitive streak at the posterior pole. (C) Midstreak stage embryo at 6.5–7.0 dpc, with the amniotic fold pushing across the proamniotic cavity. Intracellular spaces within the extraembryonic mesoderm coalesce to form the extraembryonic coelom. (D) A 7.5-dpc embryo with fetal membranes and allantois showing the left lateral wing of the embryonic mesoderm, which spreads between the epiblast and the visceral endoderm. (E) Headfold stage embryo at 8.5 dpc, showing embryonic and extraembryonic mesoderm. In parts B–E, stippled area represents the mesoderm, and triangles indicate the boundary between the embryonic and the extraembryonic regions. In parts C–E, the placenta and parietal yolk sac, which would surround the entire embryo, are not shown. 274 P436643-V1-24 30/07/04 1:01 AM Page 275 24. Early Embryonic Mesoderm Development Epiblast cells mostly converge on the streak, undergo the epithelial to mesenchymal conversion, ingress, and then move away from the streak between the epiblast and the endoderm. However, during the life of the streak, there is evidence that some cells have properties of stem cells for a limited time. In the anterior streak, fate mapping studies have indicated that some cells not only have progeny that differentiate as mesoderm and move away from the streak but also have progeny that remain in the streak, continuing to self-renew and retaining the potential to form new mesoderm.3 Fate Map of the Primitive Streak Different types of mesoderm begin to appear upon departure from the primitive streak. The timing and order of their appearance is a closely coordinated choreography that has been revealed mostly by fate mapping studies relying on cell grafting or marking and short-term embryo culture (reviewed by Lawson4 and Tam and Quinlan5). The differentiation of the different types of mesoderm into morphologically and functionally distinct cell types is presaged by differential gene expression along the length of the streak.6 Although there is considerable overlap at the edges of the fate map boundaries, different parts of the primitive streak produce different types of mesoderm (Fig. 24–2), and the types change over time.7 The first mesoderm to emerge is the extraembryonic mesoderm from the posterior part of the early streak followed by the cardiac and cranial mesoderm from a more anterior part of the steak. The extraembryonic mesoderm pushes across the proamniotic cavity, carrying with it the overlying layer of extraembryonic ectoderm (Fig. 24–1C). As it does so, intercellular cavities form within the layer and coalesce into the extraembryonic coelom, effectively separating the mesoderm into two layers. This extraembryonic mesoderm will form the yolk sac mesoderm and the mesoderm of the amnion and chorion. Later, the allantois will bud out of the most posterior part of the streak and move across the extraembryonic coelom Extraembryonic mesoderm Lateral plate mesoderm Ectoderm Primitive streak Somites Notochord/head process Node Figure 24–2. Representative fate map of the mid- to late streak stage epiblast of the mouse embryo. The differently shaded areas represent the progenitors of the tissues indicated in the epiblast prior to ingression through the node or streak. The boundaries are not as sharp as indicated; rather, they have considerable margins of overlap. to establish a connection with the chorion (Fig. 24–1D), forming the umbilicus. The cranial and the cardiac mesoderm push anteriorly around the egg cylinder in the embryonic region, eventually meeting in the anterior midline. The rest of the embryonic mesoderm arises from the middle to the anterior part of the streak, producing first the lateral plate mesoderm then the intermediate and paraxial mesoderm from the midstreak region. The axial notochord arises from the node as does the head process, an anterior extension of the streak. The node contributes cells to the cranial mesoderm and the most anterior somites. As the streak regresses, production of extraembryonic mesoderm ceases, the notochord continues to be formed from the regressing node, and paraxial presomitic mesoderm continues to be produced. By the time the tail bud takes over the production of mesoderm, only lateral plate, somitic mesoderm, and notochord are being produced.8 Extraembryonic Mesoderm The extraembryonic mesoderm remains closely associated with both the ectoderm and endoderm layers in the extraembryonic region, but in so doing it splits into two layers, the somatic and splanchnic layers, by the formation of a cavity between them known as the extraembryonic coelom or exocoelom. Combined with ectoderm, somatic mesoderm makes up the amnion, which remains as a nonvascular, protective membrane surrounding the fetus. Extraembryonic mesoderm makes up the mesothelial lining of the chorion, which, combined with trophectoderm derivatives, will later take part in the formation of the chorioallantoic placenta. Combined with the extraembryonic endoderm, splanchnic mesoderm makes up the yolk sac, which will form vascular endothelial cells throughout that will eventually coalesce into the vitelline circulation (Fig. 24–1D). In egglaying vertebrates, extraembryonic mesoderm is also associated with endoderm in the allantoic sac, whereas in mammals such as mouse and human, the allantois is almost purely mesodermal with only a rudimentary endodermal component, as the allantois has lost its role as a waste retention sac. In placental mammals, the primary function of the allantois is to provide the vascular, umbilical link between mother and fetus, a role fulfilled by the mesoderm on its own. Within the yolk sac mesoderm, vasculogenesis takes place in intimate association with hematopoiesis, or the formation of blood cells, in specialized areas called blood islands. It is here that the elusive hemangioblast is thought to reside. The hemangioblast is a common progenitor of both endothelial and hematopoietic lineages and may also be the precursor of vascular smooth muscle cells, qualifying it as a stem cell for the vascular and hematopoietic lineages.9–11 From the yolk sac blood islands, as well as from other extraembryonic areas such as the vitelline and umbilical arteries, the hematopoietic stem cells (HSC) arise and function for a limited period during mid- to late gestation, producing the primitive blood cell lineages and seeding the embryonic hematopoietic system.12 The embryonic hematopoietic system has its origin in multiple independent sites, including some intraembryonic sites (see the next section). 275 P436643-V1-24 30/07/04 1:01 AM Page 276 Virginia E. Papaioannou Embryonic Mesoderm In the embryonic region of the gastrulating mouse embryo, the mesoderm moves away from the primitive streak and spreads in an anterior direction between the endoderm and epiblast layers as two lateral spreading wings of mesenchymal cells. These wings eventually meet in the anterior part of the embryo, thus forming a continuous sheet of mesoderm between the endoderm and the epiblast, which has begun to differentiate as the ectoderm layer of the embryo. At the same time, the node generates the head process, which moves anteriorly in the midline, and the notochord, which forms the axial supporting rod of the vertebrate embryo from the level of the developing forebrain to the tip of the tail. Along the length of the embryonic axis, the mesoderm that ingresses first through the primitive streak moves the farthest from the midline and differentiates as the lateral plate. In a manner similar to the formation of the extraembryonic coelom, this mesoderm splits into two layers by the coalescence of intercellular cavities to form the intraembryonic coelom. The layers are continuous with the extraembryonic somatic and splanchnic layers of mesoderm, and the cavity is continuous with the extraembryonic coelom. The mesoderm that ingresses next and remains closer to the midline is called the intermediate mesoderm; that ingressing later and remaining closest to the midline is known as the paraxial or somitic mesoderm. The three subdivisions— lateral plate, intermediate, and paraxial—along with the axial notochord continue to be formed progressively in an anteriorto-posterior direction as the primitive streak regresses posteriorly. Lateral plate, paraxial, and axial mesoderm are produced by the tail bud toward the end of gastrulation to make up the mesodermal structures of the tail. Gastrulation, during which the endoderm as well as the mesoderm is produced by ingression through the primitive streak, is a dynamic process, proceeding in an anterior-toposterior progression. While the more posterior mesoderm is still young, the more anterior mesoderm has already begun differentiating, taking on specific mesodermal identities commensurate with its axial level and position in the embryo. The morphological subdivisions of mesoderm—cranial, cardiac, paraxial, axial, lateral plate, and intermediate mesoderm—all have distinct fates, and most interact with endoderm- or ectoderm-derived tissue during subsequent stages of organogenesis. In addition, mesoderm throughout the embryo forms endothelial cells as the basis for the circulatory system and the definitive HSC arise from embryonic mesoderm from the aorta–gonad–mesonephros region,13 providing blood cells in addition to those derived from the extraembryonic region to seed the fetal liver and eventually the adult sites of hematopoiesis: the thymus, spleen, omentum, and bone marrow. CRANIAL AND CARDIAC MESODERM In the head region, unlike other regions of the embryo where bone is derived exclusively from mesoderm, the bones of the cranium, face, and neck are formed from ectoderm-derived neural crest cells as well as mesoderm. The muscles of the face and neck, with the exception of the iris muscles, are derived from the anterior paraxial mesoderm. The cardiac mesoderm progenitors are initially in the most anterior region of the gastrulating embryo within the splanchnic mesoderm layer, but they come to lie in a ventral position caudal to the cranial region with the formation of the anterior head fold and the lateral body folds, which shape the embryonic body (Fig. 24–1E). The pericardial cavity, within which the heart develops, forms by the coalescence of extracellular spaces between mesoderm cells as the endothelial heart tubes come together to form the primitive heart tube. Although the heart is an exclusively mesoderm-derived tissue, it is thought that its differentiation is induced by the associated anterior visceral endoderm (see Schultheiss and Lassar14 for review). As the vascular network forms throughout the mesoderm of the embryo, it connects with the pulsating heart and with the extraembryonic vascular network, eventually forming a complete circulatory system with intraembryonic and extraembryonic components. L ATERAL PL ATE With the formation of the anterior, posterior, and lateral body folds, the somatic and splanchnic layers of mesoderm with their associated ectoderm and endoderm, respectively, gradually gather on the ventral side of the embryo to meet at the umbilicus. This closes the connection between the extraembryonic coelom and the intraembryonic coelom, which later forms the abdominal and pleural cavities within the embryo. Thus the somatic and splanchnic mesoderm layers of the lateral plate completely line the body cavities and form the serous membranes. In association with the endoderm, the splanchnic mesodermal layer of the lateral plate also takes part in the formation of the gut-associated derivatives of the respiratory and digestive systems: It forms the vascular components, the supporting mesenteries, and the muscular components of the gut along the length of the gut tube from the esophagus to the colon. From its position surrounding and supporting the gut, it also participates in the organogenesis of gut outpocketings forming the connective and stromal tissue of organs such as the trachea and lungs, the liver, and the pancreas. The somatic mesoderm of the lateral plate, in association with the overlying ectoderm, forms the ventrolateral body wall, including the ventrolateral dermis. This somatic mesoderm is also the source of body wall muscles and the cells that ossify into the sternum. In addition, the lateral plate mesoderm forms the two pairs of limb buds that push out from the ventrolateral body wall. Complex interactions take place between the lateral plate mesoderm and the overlying ectoderm to form and pattern the limbs.15,16 The bones of the limb develop from the lateral plate mesoderm, whereas cells migrate into the developing limbs from the somites to form the limb musculature. INTERMEDIATE MESODERM The intermediate mesoderm comes to lie in parallel ridges in the roof of the intraembryonic coelom on either side of the 276 P436643-V1-24 30/07/04 1:01 AM Page 277 24. Early Embryonic Mesoderm Development midline in the thoracic and abdominal regions. These ridges, known as the urogenital ridges, later form both the excretory and the reproductive organ systems.17,18 The development of these two systems is closely interconnected. Early in development, the intermediate mesoderm in the extreme cranial end of the embryo forms a vestigial and transitory excretory system, the pronephros. Later, in the thoracic region, the mesonephros and mesonephric ducts form in the urogenital ridge. The mesonephros is transitory, but the mesonephric duct persists in male embryos and becomes part of the genital system. The definitive kidney, or metanephros, is induced in posterior intermediate mesoderm by an outgrowth of the mesonephric duct called the ureteric bud. The gonads arise from the medial portion of the urogenital ridges from swellings called the genital or gonadal ridges, although the primordial germ cells (PGCs), the stem cells for oogonia and spermatogonia, arise from a distant site. The PGCs first appear in the posterior part of the primitive streak, migrate into the base of the allantois, then reenter the embryo to migrate along the dorsal mesentery and eventually into the gonads.19 PARAXIAL MESODERM Immediately adjacent to the axis, flanking the neural tube and notochord is the paraxial mesoderm, which will form the transitory, segmental blocks of tissue called the somites (Fig. 24–1E). Throughout the process of gastrulation, the paraxial mesoderm is continuously ingressing, segmenting to form somites, and differentiating in an anterior-to-posterior progression so that by the time the tail somites are newly formed, the most anterior somites have already undergone differentiation into other structures. The regular segmentation of presomitic paraxial mesoderm involves a molecular oscillator, called the segmentation clock, which utilizes the conserved Notch signaling pathway.20 After the initial epithelialization into segmental blocks, the somites rapidly undergo regional diversification into the dorsolateral dermomyotome and the ventromedial sclerotome. As the name implies, the dermomyotome further differentiates into the dermal component of the skin of the dorsolateral body, and into skeletal muscle, including the muscle of the limbs.21,22 The sclerotome undergoes a further transition to mesenchymal cells and resegments around the neural tube to form the vertebrae of the axial skeleton. The resegmentation of sclerotome to form vertebrae is out of register with the original somite segmentation such that cells from the anterior part of one somite combine with cells from the posterior part of the preceding somites to form a vertebral segment. In their differentiation, the somites are subject to the influences of multiple signals from various signaling pathways emanating from adjacent tissues. For example, Sonic hedgehog signals from the notochord and floor plate of the neural tube induce sclerotome differentiation, signals from the dorsal neural tube act to delimit the dermomyotome (Wnt signals) and dermis (neurotrophin 3), and Wnt and inhibitory bone morphogenetic protein signals from the body wall and mesoderm, respectively, act together on the dorsolateral somite to induce limb and body wall musculature.22–24 NOTOCHORD The axis of the embryo is defined by the position of the primitive streak at the posterior pole, with the epiblast dorsally located and primitive endoderm ventrally located. Cells that ingress through the node at the anterior end of the primitive streak and move cranially differentiate as a solid rod of cells called the notochord. These cells progress as far as the precordal plate, an area near where the buccopharyngeal membrane will later open as the mouth. As the primitive streak regresses in later gastrulation, notochord cells continue to ingress, forming a solid rod of mesoderm along the axis of the entire trunk and tail. It is around this structure that sclerotome-derived cells condense to form the vertebrae. The notochord makes only a small cellular contribution to the adult vertebral column, persisting only in the intervertebral disc. However, it is an important signaling center and structural component during embryogenesis of all chordate embryos. ACKNOWLEDGMENTS I wish to acknowledge support from the NIH (GMO 60561 and HD33082). REFERENCES 1. Sadler, T.W. (2000). “Langman’s Medical Embryology,” 8th ed. Lippincott, Williams & Wilkins, Philadelphia. 2. Beddington, R.S.P. (1994). Induction of a second neural axis by the mouse node. Development 120, 613–620. 3. Lawson, K.A., Meneses, J.J., and Pedersen, R.A. (1991). Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 113, 891–911. 4. Lawson, K.A. (1999). Fate mapping the mouse embryo. Int. J. Dev. Biol. 43, 773–775. 5. Tam, P.P.L., and Quinlan, G.A. (1996). Mapping vertebrate embryos. Curr. Biol. 6, 104–106. 6. Nagy, A., Gertsenstein, M., Vintersten, K., and Behringer, R. (2003). “Manipulating the Mouse Embryo: A Laboratory Manual,” 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 7. Tam, P.P.L., and Beddington, R.S.P. (1987). The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development 99, 109–126. 8. Tam, P.P.L., and Tan, S.S. (1992). The somitogenetic potential of cells in the primitive streak and the tail bud of the organogenesisstage mouse embryo. Development 115, 703–715. 9. Fehling, H.J., Lacaud, G., Kubo, A., Kennedy, M., Robertson, S., Keller, G., and Kouskoff, V. (2003). Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130, 4217–4227. 10. Choi, K., Kennedy, M., Kazarov, A., Papadimitriou, J.C., and Keller, G. (1998). A common precursor for hematopoietic and endothelial cells. Development 125, 725–732. 277 P436643-V1-24 30/07/04 1:01 AM Page 278 Virginia E. Papaioannou 11. Ema, M., and Rossant, J. (2003). Cell fate decisions in early blood vessel formation. Trends Cardio. Med. 13, 254–259. 12. Galloway, J.L., and Zon, L.I. (2003). Ontogeny of hematopoiesis: examining the emergence of hematopoietic cells in the vertebrate embryo. Curr. Topics Dev. Biol. 53, 139–158. 13. Dzierzak, E. (2003). Ontogenic emergence of definitive hematopoietic stem cells. Curr. Opin. Hematol. 10, 229–234. 14. Schultheiss, T.M., and Lassar, A.B. (1999). Vertebrate heart induction. In “Heart Development,” (R.P. Harvey et al., eds.), pp. 52–62. Academic Press, San Diego. 15. Niswander, L. (2002). Interplay between the molecular signals that control vertebrate limb development. Int. J. Dev. Biol. 46, 877–881. 16. Tickle, C. (2003). Patterning systems—from one end of the limb to the other. Dev. Cell 4, 449–458. 17. Dressler, G.R. (2002). Development of the excretory system. In “Mouse Development: Patterning, Morphogenesis, and Organogenesis,” (J. Rossant et al., eds.), pp. 395–420. Academic Press, San Diego. 18. Swain, A., and Lovell-Badge, R. (2002). Sex determination and differentiation. In “Mouse Development: Patterning, Morphogenesis, and Organogenesis,” (J. Rossant et al., eds.), pp. 371–393. Academic Press, San Diego. 19. Anderson, R., Copeland, T.K., Scholer, H., Heasman, J., and Wylie, C. (2000). The onset of germ cell migration in the mouse embryo. Mech. Dev. 91, 61–68. 20. Pourquie, O. (2001). The vertebrate segmentation clock. J. Anat. 199, 169–175. 21. Buckingham, M., Bajard, L., Chang, T., Daubas, P., Hadchouel, J., Meilhac, S., Montarras, D., Rocancourt, D., and Relaix, R. (2003). The formation of skeletal muscle: From somite to limb. J. Anat. 202, 59–68. 22. Duprez, D. (2002). Signals regulating muscle formation in the limb during embryonic development. Int. J. Dev. Biol. 46, 915–925. 23. Gossler, A., and Hrabe de Angelis, M. (1998). Somitogenesis. Curr. Topics Dev. Biol. 38, 225–287. 24. Summerbell, D., and Rigby, P. W. (2000). Transcriptional regulation during somitogenesis. Curr. Topics Dev. Biol. 48, 301–318. 278