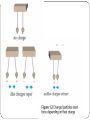
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Science 9 electricity powerpoint Topic 1
Carbon nanotubes in photovoltaics wikipedia , lookup
Opto-isolator wikipedia , lookup
Electrical engineering wikipedia , lookup
Rectiverter wikipedia , lookup
Surge protector wikipedia , lookup
Electric battery wikipedia , lookup
Nanogenerator wikipedia , lookup
Rechargeable battery wikipedia , lookup
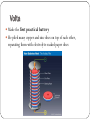
Science 9 Unit D Section 1.0 Electrical Principles & Technologies 1.1 – Static Electricity Static electricity is produced when two objects rub together This produces a buildup of charge on the objects being rubbed together http://www.youtube.com/watch?v=fT_LmwnmVNM How Static Electricity works The Atom Protons have a POSITIVE CHARGE Electrons have a NEGATIVE CHARGE Electrical Charge Positive and negative charges on an object are due to the ratio of protons and electrons present in an object Objects are negative when they have more electrons than protons Objects are positive when they have more protons than electrons Objects are neutral when the amounts of positive and negative charges are equal Producing a Charge Charges are produced when electrons move from one object to another An object that loses electrons becomes positively charged while one that gains electrons becomes negative Only the electrons move, never the protons! Charge Separation Charged objects cause charge separation when they are brought near to neutral objects Electrical Discharge Static electricity generally does not move – it remains stationary (or is static) However, a built-up static charge on an object can be attracted to other objects and jump to the object Often this creates a small spark, which is known as an electrical discharge Laws of Electrical Charges Opposite charges attract each other Like charges repel each other Neutral objects are attracted to charged objects Van de Graaff Generators A Van de Graaff Generator is used to study electrical charges A rubber belt running in the generator rubs against metal “brushes” and creates a static charge The charge is then transferred to the metal sphere, where the static charge builds up Mythbusters Van de Graaff generator http://www.youtube.com/watch?v=7qgM1A3pgkQ 1.2 – Current Electricity Static charges do not work very well for operating electrical devices Electrical current is produced as charges move in a continuous flow An electrical current flows until it is used up or disconnected Think of it like water flowing until it is dammed or its source drives up Electric Eels http://www.youtube.com/watch?v=a3sviTa8hZw World’s deadliest: Six-Foot Electric Eel Amperes The rate at which current flows is measured in amperes (A) Called Amp for short Named after Andre-Marie Ampere Electrical current refers to the number of electrons passing a point in a circuit per second How do we move electricity to its destination? Answer: CONDUCTORS Conductors are materials that electrical energy can move easily through Circuits A circuit is a path that controls the flow of electricity (just like pipes and taps in a water system control the flow of water) In most pathways, electricity flow along solid metal wires, but circuits can also include gases, other fluids, etc Parts of a Circuit A complete electrical circuit consists of: LOAD: a device that converts electrical energy to another form (ex. A lightbulb) ENERGY SOURCE: source of electrical energy (ex. Battery) CONDUCTOR: pathway for electricity to flow (ex. Wires) Electrical Energy & Voltage Voltage is the measure of the amount of electrical energy carried by each particle Voltage is also known as “potential difference” The unit for voltage is known as the Volt (V) Voltmeters are used to measure voltage Measuring Voltage When setting up a voltmeter, the red lead must run to the positive battery terminal, and the black lead should run to the negative terminal 1.3 Electrical Safety Electricity has the ability to seriously injure or kill This is because electrical current can interrupt the function of the nervous system and heart (both of which rely on their own electrical current to operate) Short Circuit A short circuit is an unintended pathway for electricity The current bypasses part of the normal circuit Electrical Shock High voltage is more likely to cause damage than low voltage However, the number of amps is much more significant This is because you may only have a few high-energy electrons flowing through you if there is a high voltage and a low current However, high currents mean that lots of electrons are flowing through your body Current Effects 0.001 A: 0.015 A: 0.100 A: Effects are likely not felt Painful shock and loss of muscle control Fatal Protecting From Shock Insulators prevent the movement of electrical current and will protect you from electrical shock Current does not move freely through insulators such as wool, rubber, and air. Electrical Safety Pointers Plugs, Fuses & Breakers The third prong on electrical plugs is the ground This provides a route through which excess charge can escape to the ground (instead of building up and discharging through you!) Plugs, Fuses & Breakers Both fuses and circuit breakers limit the amount of current flowing through a circuit If a fuse or breaker receives too much current, the circuit is broken and the current stops Lightning The current in a lightning strike can be as high as 30 000 A To avoid being hit by lightning, avoid the following: - standing on hilltops or under trees - standing near metal objects Lightning Rods Lightning rods are often added to tall building peaks They are connected to the ground with wires Any discharge from a lightning strike is conducted harmlessly to the ground (instead of destroying the building or its wiring) 1.4 – Cells and Batteries An electrochemical cell is a device that provides a steady current that is produced by a chemical reaction Some people have problems with the electrical signal that controls beating of the heart, a PACEMAKER can be surgically inserted to send small amounts of current to keep the heart beating normally Electrochemical cells come in two types: Wet cells and dry cells Dry Cells A dry cell is an electrochemical cell that uses chemicals in a paste form The cell consists of two different metals and an electrolyte http://www.youtube.co m/watch?v=KkRwuM4S 8BQ Dry Cells - Terms An Electrolyte is a paste or liquid that conducts electricity An Ion is an atom or group of atoms that has come electrically charge through the loss or gain of electrons An Electrode is a metal that the electrolyte reacts with Wet Cells A wet cell uses an aqueous solution as the electrolyte Wet cells are less common than dry cells Why? They are more expensive, not as reliable and can leak Chemical Reactions & Electrochemical Cells In a wet or dry cell, the electrolyte reacts with the two metals One metal loses electrons and becomes positively charged The electrons are passed through the solution and are deposited on the other metal, which becomes negatively charged When connected to a circuit, the electrons leave the negative electrode and move through the circuit to the positive electrode Rechargeable Cells Some dry and wet cells are primary cells (they cannot be recharged) Secondary cells (which are rechargeable) can regain stored charge if an electrical current is run “backwards” through the cell These cells use chemical reactions that can be relatively easily reversed Batteries A battery is a collection of cells connected together “AA”, “AAA”, “C” and “D” type cells are not batteries – they are single electrochemical cells Larger batteries such as 9-V and 12-V batteries are true batteries Volta Made the first practical battery He piled many copper and zinc discs on top of each other, separating them with electrolyte-soaked paper discs Electrolysis Electrolysis is a technique that is used to break free atoms from compounds This is done by running a current through the substance For instance, electrolysis can be used to produce oxygen and hydrogen from water Or separate pure metals from molten compounds Electrochemistry Is the study of chemical reactions involving electricity Electroplating Electroplating A thin layer of metal can be plated onto a cheaper metal using electricity The item to be plated is immersed in an electrolyte along with an electrode that consists of the metal that will be used for plating Electricity flows from the plating metal to the object being plated, and as a result the metal moves across the solution and builds up on the surface of the object being plated Other Applications of Electrochemistry Anodizing – aluminum is coated with a layer of aluminum oxide, which is much harder than regular aluminum Electrorefining – impurities are removed from impure metals by placing the metal in an acid and running a current through it