* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Collaborative action research to reduce patient waiting times
Survey
Document related concepts
Transcript
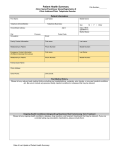
Manuscript ic r t n a Manuscript astr ta ait ti r su ts su issi n c Collaborative action research to reduce patient wait times: Results in two high-demand public clinics in Western Cape, South Africa Short title: Action research to reduce patient waiting in South Africa M. Anjali Sastry1,2*, Katelyn N.G. Long1,3, Angela de Sa4,5, Haniem Salie4, Stephanie M. Topp6,7,8, Saurabh Sanghvi1, Lindi van Niekerk9 1. Sloan School of Management, Massachusetts Institute of Technology, Cambridge, Massachusetts, United States of America 2. Department of Global Health and Social Medicine, Harvard Medical School, Boston, Massachusetts, United States of America 3. School of Public Health, Boston University, Boston, Massachusetts, United States of America 4. Western Cape Department of Health, Western Cape, South Africa 5. Health Sciences Faculty, Division of Family Medicine, University of Cape Town, Cape Town, South Africa 6. Centre for Infectious Disease Research in Zambia, Lusaka, Zambia 7. School of Public Health and Medicine, University of Alabama at Birmingham, Alabama, United States of America 8. Nossal Institute for Global Health, University of Melbourne, Victoria, Australia. 9. Bertha Centre for Social Innovation and Entrepreneurship, Graduate School of Business, University of Cape Town, Cape Town, South Africa * corresponding author. email [email protected] Abstract Introduction: Lengthy waiting times are problematic for patients and healthcare workers alike. In clinics and hospitals across Africa, persistently long wait times have been linked to poor medication compliance, skipped appointments, delayed implementation of clinical programs, and low healthcare worker morale. This paper presents results from collaborative action research projects at two high-demand primary care public health facilities in Cape Town, South Africa. Projects were built around two-week focal interventions undertaken by clinic staff and US-based graduate students in concert with clinic leadership, researchers, and faculty. Collaborators aimed to generate practical local solutions to improve within-facility wait times. Methods: Drawing on detailed time stamp data for over 2,400 patient visits, we compared entry-to-exit times before and after focused action research collaborations and examined operational changes along with sitespecific enabling and constraining factors. Findings: Mean wait times were significantly reduced, from 129 to 102 minutes in one clinic’s pharmacy and from 275 to 196 minutes in the second clinic. Operational changes sustained over three months after the focal collaboration took the form of procedural improvements, technology adjustments, tailoring of patient visits, and team capacity-building. Contextual barriers included staff turnover, varying staff participation, mandated software changes, and resource constraints, while enablers included leadership commitment, experience with past improvement collaborations, and advocacy. Conclusions: Amid the complexity of operating a busy primary care facility and an array of structural and relational factors, the action research collaboration demonstrated that simple changes generated in situ can help address the vexing challenge of persistent lengthy wait times. Further research is needed to assess ongoing sustainability and broader applicability, but results underscore the potential value in enabling facilities to collect and analyze operational data. Our findings reveal the promise of short term, inclusive action research 1 collaboration involving frontline health workers, facility leadership, and targeted external support to trigger potentially beneficial change. 1. Introduction Long patient wait times are a persistent challenge facing healthcare systems across Africa, and have been linked to a variety of undesirable outcomes [1-7]. At the primary care level, where patient adherence is an ongoing concern, longer waits are associated with lower levels of patient satisfaction, reduced willingness to return, more missed appointments, poorer ratings of clinical providers, and inappropriate self referral to higher level facilities [8-14]. In the short run, excessive waiting increases the likelihood that patients will leave without being seen, and can compromise patient safety [15-16]. The length of time spent standing in clinic queues, tracking down paperwork or staff, and waiting for medication are frequently recorded causes of patients dropping out of antiretroviral treatment (ART) programs [1-2]. In Uganda, Tanzania, and Botswana, lost wages linked to lengthy clinic visits present a major obstacle to optimal ART adherence [3]. One study in Zimbabwe found that patients had a “sense of fear and dread” of long queues, which often necessitated scrambling to keep their place in line and caused worry about not seeing the clinician before the day’s end [6]. In Nigeria, a study of an outpatient department showed that the majority of patients were dissatisfied with services, due mostly to long waiting times. Despite nearly 80% of patients reporting that they felt wait times should not exceed 30 minutes, a majority of patients waited far longer, with those waiting more than 60 minutes expressing higher levels of dissatisfaction [7]. Wait times are difficult to manage in primary care facilities, owing to their lack of buffers and tight coupling [17-18], but a growing number of studies have laid the groundwork by tracking patient flows and measuring waiting. In sub-Saharan Africa, within-facility mean wait times of four hours or more are not unusual (for instance, wait times averaged 3.8 – 4.6 hours in three clinics in Uganda [19] and 6.5 hours at an ARV clinic in Nigeria [7]). Excessive extreme waits exacerbate the challenge: in an ARV clinic in Botswana, mean wait times were four hours but some patients waited 12 hours [3]; in a study of patient experience at a teaching hospital in Benin, Nigeria, the average wait was 2.9 hours, but individual waits ranged from two minutes to two days [20]. In sub-Saharan Africa, long wait times are often symptomatic of low provider-to-patient ratios that contribute to health workers’ inability to spend appropriate time attending to each patient. In a study exploring moral distress of nurses at high volume facilities in Malawi, one participant reported that the daily strain of attending to as many patients as possible and still not being able to care for everyone in the queue left her feeling sad and alone [21]. Long wait times and the factors that cause them are thus closely associated with low 2 staff morale. Excessive wait times and their attendant disorder may also undermine other micro-level system initiatives, such as the integration of routine primary level outpatient and ART services [22]. Complaints about staff fairness and bribe-taking may accompany patients’ experience of excessive waiting [23]. To enable practical efforts to reduce patient waiting times in two busy urban facilities, an action research collaboration was formed between the Western Cape Department of Health, the Bertha Centre for Social Innovation and Entrepreneurship at the University of Cape Town Graduate School of Business, and the Sloan School of Management at the Massachusetts Institute of Technology (MIT). The research was built around projects undertaken in a selective MIT Sloan course offered to graduate students with an average of six years of professional experience in management consulting, engineering, financial services, software, and other fields. The course, called Global Health Lab, enabled collaborative improvement projects related to healthcare delivery in low-resource settings in Africa and Asia. Projects were designed and executed by a team of MIT students, staff, and faculty working jointly with healthcare organizations to apply management tools and approaches to challenges in operations, strategy, marketing, or technology adoption (see groundwork.mit.edu for more). In late 2013, leaders of two high-demand public primary care clinics in Cape Town proposed Global Health Lab projects that would identify bottlenecks and test low cost, locally designed innovations in operations to reduce patient waiting times. The wait time study presented here was part of a broader action research effort to examine the facilities’ experience with their projects. In this paper we compare time spent by patients from entry to exit at each clinic before and after the project, discuss operational and other changes, and explore sitespecific factors that both enabled and constrained potential improvements. 1.1 Research Setting South Africa’s leaders have long been committed to improving public health services [24-26]. But despite spending 8.6% of gross domestic product on healthcare—on par with England, Spain, and Italy—the country’s health system performance has fallen short of desired health impacts [27]. Growing burdens of disease, deep socioeconomic disparities, a rising population of refugees, and lingering effects of the country’s inequitable past have all contributed to suboptimal health system performance [28-29]. In addition, human resource constraints mean that South Africa’s public sector has had to serve up to 84% of the population with only 30% of the country’s medical doctors. The country’s annual per capita spending on healthcare for privately insured citizens is $1400, compared with $140 for those served by the public sector [29]. Observers have variously attributed a crisis in public facilities to a combination of poor infrastructure, limited governance, and managerial capacity [29-30], along with discrepancies between top-level developments and ground-level adoption [27]. The need for change has been recognized at the highest levels. In 2014, South Africa’s Minister of Health Dr Aaron Motsoaledi made the case for tackling the persistent challenge of service delivery improvement: “Innovation has occurred in drugs, diagnostics, therapeutics, and devices, but now, more than 3 ever, innovation is required in healthcare delivery systems. An example of this pressing need is prolonged patient waiting times” [31]. With a population of 5.9 million [32], the province of Western Cape is South Africa’s fourth most populous. Today it faces demographic trends that include an aging population, a changing racial mix, and growing urbanization and migration, particularly in Cape Town, the province’s largest city [33]. In terms of performance and resources, the Western Cape health system is compared favorably to the rest of South Africa [34], even as it works to respond to recent growth in the burden of non-communicable disease, high rates of tuberculosis and HIV, and multiple unmet needs for maternal and child health along with injuries to due violence and road traffic accidents. In the Cape Town metropolitan area, the public health system is composed of 46 provincially managed primary healthcare facilities that provide care to the uninsured population across eight sub-districts, each with a district hospital and a mix of 24-hour and 8-hour facilities. As a key element of its 2030 vision, the Western Cape Department of Health called for waiting times to be brought to levels that would be acceptable to patients [33]. Several provincial healthcare facilities responded by launching efforts to improve to reduce waiting times and improve patient experience via lean initiatives and other change interventions [35]. Two provinciallymanaged primary care facilities in Cape Town, which we call “A” and “B,” embarked on a novel approach to address wait times by requesting work collaborations with GlobalHealth Lab. Clinic A was a 24-hour comprehensive public primary care facility providing an extensive set of generalist acute and chronic services that included HIV, maternity, and emergency care. The clinic’s 118 staff served an average of 18,000 patients per month; on some days, up to a thousand patients could be seen. In proposing a GlobalHealth Lab project, Clinic A’s leadership sought to tackle a particularly challenging issue: the length of time that patients waited within its busy pharmacy (which served patients from all clinic departments) during the hours of peak operation, 8:00 am – 5:00 pm. Patient visits to the pharmacy fell into three main categories. For “acute” visits, patients were seen by a doctor or clinical nurse practitioner without a previously scheduled appointment and then sent to pharmacy for medications. Chronic dispensing unit (“CDU”) visits involved patients registered at the clinic for ongoing care for certain chronic conditions, such as diabetes, who came directly to the pharmacy to pick up preassembled medication parcels. “Repeat” visits were for patients who had appointments to pick up medication but were not in the CDU system. Flows of patients, information, and medications within the pharmacy varied by visit type and patient needs, as Figure 1 shows. Figure 1: Overview of the processes in Clinic A pharmacy For Acute and Repeat patients, folders were taken at a pharmacy window and placed in a queue until patient data were entered into the electronic pharmacy record system (EPRS). Once entered into EPRS, folders waited in the picking queue until a staff member pulled medication from the shelves. Once picked, medications were placed in another queue until the pharmacists dispensed them to patients. CDU patients followed the same general process using a designated window for 4 their patient cards to be taken in and for medication to be dispensed in pre-packaged bundles. During the entire process, all patients waited in a seating area just outside the pharmacy. Clinic B was an 8-hour public primary care facility providing services five days a week. Its staff of 42 served an average of 13,000 patients per month with comprehensive services that included basic antenatal care, reproductive health, chronic disease management, diagnostic and wellness services, and other curative care, mental health, oral health, occupational therapy, physiotherapy, and casualty services. Patient visits at Clinic B involved “booked” patients who had appointments to see a doctor, “unbooked” patients who did not have an appointment, and “refill” patients who went directly to the pharmacy to pick up pre-filled prescriptions. Clinic B’s leaders sought to improve patient flow throughout the clinic and reduce the overall length of time that all patients waited from entry to exit. The project initially focused on the process and system in the clinic’s reception area, but soon expanded to include various parts of the clinic operations that had an impact on patient flow from entry to exit (Fig. 2). Figure 2: Overview of the processes in Clinic B Patients sat in the arrival waiting area until called to the appropriate registration window for booked, unbooked, or pharmacy refill visits. Refill patients proceeded directly to the pharmacy following registration; booked patients waited to be called to the preparation room for measurements of vital signs, and unbooked patients waited to be called into a sorting room to determine if they needed to see a doctor or make use of another service offered at the clinic. If a doctor visit was needed, unbooked patients proceeded to the preparation room. Following sorting and preparation visits, patients waited once more outside the doctor’s office, then again in the pharmacy until their names were called for medication dispensing. 1.2 The Broader Action Research Study We employed action research to ensure that the wait time improvement efforts were contextualized and customized for each clinic’s needs—and appropriately studied and understood. In the Western Cape, action research addressing governance in primary care has underscored the importance of understanding frontline implementation [36-38]. The method helps participants to comprehend an organizational or social system from an integrated perspective while they simultaneously engage with each other to improve it in some way [39-41]. Its linked cycles of planning, action, and evaluation guide researchers to actively explore current practices in concert with stakeholders and develop shared knowledge to support and facilitate interventions or new practices [41]. The cyclical design of action research allows researchers to continually share emerging results with partners, reflect together on their interpretation, and then adapt actions to account for new insights about the needs of the system and its stakeholders [41-44]. Triangulation of data, contextualization of experience, systematic researcher reflection, member checking, and ongoing joint review of findings all help to ensure rigor and reduce researcher bias [39,41]. The collaborative action research employed these approaches in three phases of activities conducted from November 2013 to July 2014. 5 Phase one (Months 1 – 4) During phase one, projects were scoped and work plans developed. Early discussions led the research team and clinic leadership to narrow in on the goal of reducing patient wait times in both facilities. The research team conducted site visits to gain firsthand understanding of clinic processes, conduct in-depth interviews with clinic leadership and frontline staff, and begin to co-design a wait time study in each facility. Each team of four MIT graduate students also began to work closely with its assigned clinic and the research team to build their understanding of clinic processes and review the experience with comparable investigations. In the final month of phase one, baseline wait time data were collected at both facilities using route slips. Phase two (Months 5 – 6) In phase two, MIT students worked intensively for two weeks at each clinic in collaboration with its staff and with oversight from the research team. Students used baseline wait time data to develop provisional hypotheses about operational bottlenecks, identify other potential improvement opportunities, and pilot test solutions. Together, clinic staff and students gathered additional data and generated new ideas to streamline processes. At the end of the onsite period, the MIT students presented a portfolio of operational improvement proposals and reported on early implementation to clinic staff and members of the research team. After their visit, students continued to work with the clinics through email and skype for another month to tailor recommendations and refine portfolios. The research team met with students and clinic leadership to document and explore the evolution of the project and related changes taking place at each facility. Phase three (Months 7 – 9) During the last phase, regular calls and emails took place between clinic leadership and the research team to discuss changes taking place at each facility and plan the endline wait time measurement. An electronic data collection tool, described below, was developed for this purpose. A final set of site visits was needed for last round of data collection. Following data analysis, the research team met with both facilities to discuss results of the wait time study, share final updates of ongoing and new operational changes underway at each facility, and to outline next steps. Table 1 summarizes activities undertaken in each action research phase. While the present study analyzes wait times along with operational changes and contextual factors that shaped implementation, future papers are planned to examine staff experience and engagement, the study team’s action research process, and other aspects of the collaboration. 6 Table 1. Collaborative action research (planning, action, evaluation) by phase Planning Phase one Phase two Phase three Weekly meetings between research team and clinic leadership Weekly calls to discuss clinic processes, provisional hypothesis, and potential solutions Bi-monthly calls between research team and clinic leadership Iteration of route slip design and data collection methodology Action Evaluation Planning for two week onsite visit Development and pilot testing barcode scanning technology Baseline wait time data collection In depth interviews with frontline staff Implementation of operational changes during onsite collaboration Creation of comprehensive portfolio for each clinic Endline wait time data collection Evaluation of baseline wait time data Expert faculty review of final portfolio Evaluation of endline wait time data Feedback and discussion of results between research team and clinic leadership Online survey requesting feedback from clinic staff about experience with students Discussion and interpretation of results between clinic staff and research team In depth interviews with frontline staff 2 Methods To measure wait times, time-stamp data were collected in both facilities. A review of existing research on patient flows, including studies of within-facility wait times in sub-Saharan Africa [4,19-20, 22,45-50], informed our measurement approach of directly capturing the times at which each patient arrived and went through several points within each clinic (rather than relying on patient reports or clinic data alone). Route slip forms were collaboratively designed by clinic staff and the research team (see Fig. 3) to track specific times at which the patient and his or her folder entered and exited key processes. To establish our baseline measurement, when patient entered we recorded the time and visit type (e.g. acute or unbooked) on their route slip, then stapled it to their folder. The time at which patients went through each of several subsequent steps, including exit, was entered by staff and research assistants stationed throughout the clinic or pharmacy (slips contained no patient-identifying data).Watches and cell phones were coordinated to ensure accurate time reports. Clinic leadership and members of the research team monitored the process to check that data were correctly collected. Figure 3: Clinic B route slip. This is an example of the data collection form used in Clinic B. Our experience with measuring baseline patient flow timing led the clinics and research team to explore the potential for electronic data collection. The team met with local technology developers, selecting a Cape Town social enterprise [http://www.broccoliproject.org/] to prototype a new customized barcode scanning 7 system, dubbed “Scanpoint,” to capture time stamps in each patient’s journey through the clinic. After pilot testing in each facility, Scanpoint was used to collect endline time stamps. Refurbished laptop computers and bar code scanners along with custom-developed software automated the data entry that had previously relied on paper slips. As patients and their folders moved through their visits, at the points captured manually via the baseline method, research assistants and clinic staff scanned bar codes on each folder, automatically uploading identifiers, visit categories, and time stamps to an electronic database. Scanpoint stations were deployed on countertops or carts in the pharmacy and clinic (Picture 1, 2). Barcode printers generated new stickers to replace any that were unreadable. As with the paper slips, patient visit categorization took place at the first stop. Picture 1: Scanpoint deployed in a clinic. Picture 2: Another Scanpoint station. De-identified data were manually entered into Microsoft Excel for our baseline measurement; endline data were automatically uploaded into Excel and de-identified for analysis. The MIT students and research team analyzed the data, conducting wait time calculations and assessing means, medians, and standard deviation across patient categories, process times in various clinic stations, patient stock levels throughout the day, and arrival time variation. Analysis included t-tests to assess statistically significant changes in waiting time, as well as descriptive statistics such as patient volume and patient visit type. A supplemental analysis was performed using a two-sample Wilcoxon Rank Sum Test of nonparametric distribution to assess equality of medians. Baseline data were collected for three days and two days in Clinics A and B respectively; endline data were collected for two days at each facility. The timing of data collection was determined by the availability of clinic leaders and staff and researchers, as well as a variety of site-specific considerations (such as training days, implementation of new systems, and public holidays). The dataset for Clinic A addressed only the pharmacy, and excluded any other clinical or administrative steps patients took earlier in their visits. For Clinic B, our present analysis addresses length of time spent in the facility from entry to exit, not the intermediate steps within each patient visit; more granular data that tracked intermediate process timing are not examined here but were used by the action research team to generate and explore the operational changes. The research team documented each operational change, along with contextual factors, as participant-observers in the action research. In addition to baseline and endline wait time measures, both clinics collected an additional round of time-stamp data during the study period, prompted by concern that a mandated change in operations necessitated by a new electronic pharmacy records system designed for district-level record keeping would impact wait times. Methodological inconsistencies precluded these additional data from being included in the present results. Table 2 summarizes the data collected for the present study by phase. 8 Table 2. Data collected for wait time study by action research phases Data collected Phase one Phase two Phase three Process maps for each clinic Research team documentation of operational changes implemented Endline data using barcode scanning system Documentation of subsequent operational changes Baseline wait time data using paper route slips 2.1 Ethics Approval Ethics approval was obtained from the Chief Directorates, Department of Health, Western Cape Government, for Provincial Research Reference number 2014RP024; the University of Cape Town Faculty of Health Sciences Human Research Committee, IRB Protocol 642/2013; and the Massachusetts Institute of Technology Committee on the Use of Human Subjects, Protocol 1311006003. 3 Results Across the nine days of endline and baseline data collection, 3,235 patients were tracked in total at Clinic A’s pharmacy and in Clinic B. Administrative records show that the pharmacy and clinic served 3,566 patients on these five days, suggesting that 9.3% of patients were not captured by our dataset, most likely excluded in the peak arrival hours when some entering patients failed to be enrolled in the data collection process. Of the patient visits for which data was collected, a further 744 records were subsequently excluded because of missing data elements. Overall, 70% of patient visits were analyzed in the present study. In Clinic A, baseline data captured wait times for 1165 patients over three days and endline data captured wait times for 660 patients over two days. In our dataset, acute patients represented 42% and 40% of pharmacy volume in baseline and endline datasets respectively. CDU patients represented 41% and 45% respectively, and repeat patients, 17% and 15% of pharmacy volume. On baseline data collection days, ten permanent and three to eight additional staff worked in Clinic A’s pharmacy. During the endline data capture, the pharmacy was staffed by ten permanent members, with up to three additional assistants. There was a significant reduction in wait time across all three patient categories at Clinic A (p = 0.000). Overall, wait times in the pharmacy fell by 27 minutes, a 21% reduction: at baseline, mean waits measured 129 minutes, compared to an average of 102 minutes in the endline data period. For acute patients, the mean wait time was reduced from 197 minutes to 171 minutes (p = 0.003). For CDU patients, the mean wait time was halved from 66 minutes to 35 minutes (p = 0.000). Waiting for repeat patients did not change significantly (Table 3). 9 Baseline data from Clinic B captured wait times for 250 patients over two days; endline data captured wait times for 417 patients over two days. Staffing levels at endline were the same as baseline with 35 of 41 regular staff members present in the clinic. Inconsistent patient categorization on the baseline paper route slips made it difficult to compare patient volume by visit type across the two data collection periods. The electronic data collection allowed for more comprehensive patient categorization, with booked patients accounting for 27% of patient volume, unbooked patients accounting for 18%, and pharmacy refill accounting for 55% of patient volume. The reduction in patient time spent at Clinic B was also statistically significant. Overall, there was a 79minute, or a 29%, drop in mean time from arrival to departure. At baseline, patients spent an average of 275 minutes from arrival to departure, which was reduced to an average of 196 minutes by the end of the study (p = < 0.000) (Table 3). Note that in Clinic B, overall visit times included a portion of value-added time spent in clinical services. Table 3. Baseline and endline wait times at Clinic A and Clinic B Baseline Endline (N is number of patient visits; mean and median in minutes) N Mean Median N Mean Median P-value 95% CI 1165 129 86 660 102 52 0.000 (17, 38) Acute 487 197 230 262 171 177 0.003 (9, 44) CDU 484 66 43 298 35 23 0.000 (22, 41) Repeat 194 115 101 100 119 123 0.621 (-19, 11) 250 275 256 417 196 161 0.000 (55, 102) Clinic A Total Clinic B Total 4. Discussion The action research collaborations led to significant reduction in wait times in both clinics, over three months after the focal interventions took place. Our findings are consistent with a handful of other studies in sub-Saharan Africa that have tracked waiting and in-facility times before and after operational changes: a 21% drop in wait times at an urban clinic in Uganda (from 157 to 124 minutes) [46]; a 60% reduction in a community-based HIV program in Uganda (from 206 to 83 minutes) [48]; a 33% reduction in a Nigerian ARV clinic (from 390 to 261 minutes) [50]; and a 65% reduction in pharmacy waiting in Nigeria (from 157 to 55 minutes)[20]. When wait times are shortened, patients and staff may benefit from reduced crowding, greater efficiency, and less wasted time [23, 47, 51]. More patients return for follow up care and refills [52]. While 10 many observers suggest that operational improvements should reduce wait times and deliver such benefits [4, 7, 45, 53], wait time measurements and operational changes are not always systematically captured in such investigations to allow the changes to be linked to improvements in waiting. When they are, we learn that that not all changes in operations yield observed reductions in specific performance measures; for instance, in the aftermath of integrating HIV treatment into a general outpatient clinic in Zambia, mean wait times rose, which analysis revealed to be due to reductions in staff availability and longer breaks between consecutive visits [49]. The research suggests that both measurement and operational changes must be carefully documented, along with environmental factors and emerging internal responses that could influence the facility’s functioning. To put our wait time analysis in context, we begin by discussing the operational changes implemented in each clinic. We then explore site-specific factors that enabled and constrained implementation of operational changes over the course of the study period. We also examine the advantages and disadvantages of an iterative data collection methodology and address limitations of our study. 4.1 Operational changes Operational and other changes in each clinic can be grouped into four broad categories: procedural improvements, technology adjustments, tailoring patient visits, and building team capacity. The changes in each facility are discussed below. Changes in clinic A A variety of operational changes were piloted or implemented in Clinic A during phases two and three. A staff roster was developed to more efficiently assign personnel to specific tasks throughout the day based on expected patient volume and daily conditions. Five-minute morning meetings were instituted for all pharmacy staff to review daily assignments and discuss issues affecting the pharmacy. Small cardboard boxes were introduced to hold medications and patient folders in the dispensing queue, to both reduce counter clutter and improve efficiency. Brightly colored clips were added to priority folders at the pharmacy intake window to more effectively fast-track children and elderly patients. Other changes included structured efforts to increase the provision of double prescriptions, enrolling compliant patients to the more efficient CDU program, and shifting selected tasks to less-busy hours of the day to ease workloads during the busiest times. In phase three, frontline staff further refined some changes in the pharmacy. For example, to increase visibility of priority patients, the staff created cardboard signs labeled “priority” which were affixed to patient folders with the colored clips. While several operational changes were incorporated into daily practice, others were not maintained in the weeks that followed. The effort to shift selected tasks to less busy hours of the workday was deemed impractical, for example. The staff roster and the five-minute morning meetings were dropped in the weeks after the MIT students left, but near the end of phase three, a clinic administrator worked alongside pharmacy staff for a full week to support pharmacy staff efforts and reinstate the two practices; 11 months later both had become routine. At the end of the action research project, soon after the last round of data collection, clinic leadership reported the number of deferrals (patients who are asked to come back the next day to receive medication) dropped to zero from a previous daily average of 50-70. A meaningful decline in the number of patient complaints due to pharmacy wait time was also reported. Changes in Clinic B Operational changes piloted or implemented in Clinic B in phases two and three also included a range of improvements. Boxes for stray paperwork were placed throughout the facility and a simple process instituted to gather and return their contents, with the aim of addressing the previously vexing challenge of missing patient files. After the first day of implementation, the number of lost files fell by 70%. Parameters for the existing booking system in the reception area were adjusted to improve the efficiency of patient appointments. Prior to the study, gates to the clinic grounds were routinely opened an hour or more before the facility opened for the day. To reduce the morning bottleneck, the opening time of these gates was moved to 7 a.m., the established time at which clinic staff were ready to begin processing patients. In the reception area, old folders were removed and active patient folders were color-coded to improve visibility. Reception staff also began to pre-fill selected portions of patient medical forms to ease the burden on staff in other parts of the clinic. During phase three the staff formed an Improvement Team, called the “I Team,” to maintain operational changes and implement new ideas throughout the facility. Together, clinic leadership and the I Team continued to tailor planned upgrades to the booking system to increase afternoon appointment times and account for times that the clinic was short staffed (e.g. tea breaks, holidays, staff meetings). Near the end of phase three, it was Clinic B’s turn to install the new electronic pharmacy records system. The I Team created a task force to support implementation and increase staffing levels, ensuring that patient wait times would not be negatively affected by the changes in the pharmacy. Other changes generated on site that were implemented in phase three included separating phlebotomy and the emergency area, which had previously been combined; creating a new streamlined manual appointment system for patients who needed their dressings changed; and adding phone lines for improved patient communication (e.g., for blood test results). 4.2 Factors affecting implementation and impact Throughout the study period, both clinics faced an array of contextual factors, some of which enabled and others which constrained their efforts to implement operational and other changes. Factors at work in Clinic A At Clinic A, several factors enabled successful operational changes and the ensuing reduction in pharmacy wait times. First, Clinic A benefitted from dedicated leaders who championed the initiative with clinic staff to reinforce the value of reducing patient wait times, improving pharmacy processes, and supporting 12 frontline staff throughout the change process. Engaged leadership has been demonstrated to be an important factor in facility-level innovation [54-55], as leaders can actively help frontline staff make sense of new procedures and policies, engendering a sense of ownership and discretionary power over change implementation [55]. In addition, Clinic A’s clinical leader held a position within the district that enabled insights and lessons to be shared, a form of external visibility that may have contributed to commitment. Second, a previous collaboration between Clinic A and key members of the research team had fostered strong relationships and a shared experience of working together that aided data collection and documentation. The importance of such collaboration has been identified in prior action research which finds that it helps to break down inherent power imbalances and knowledge silos between “researchers” and those stakeholders traditionally viewed as “subjects” [56]. Collaborative improvement initiatives have also been linked to significant performance gains in diverse low- and middle-income country health systems, because they enable groups of stakeholders to work collectively and accelerate the pace of improvement [57]. Nonetheless, a number of factors posed implementation challenges. In the middle of phase one, Clinic A implemented the electronic pharmacy records system designed to improve district record keeping. Given the high volume of patients served by Clinic A, introducing the new software was disruptive to pharmacy operations, as it required staff to receive extra training and incorporate new processes into their daily workflows. The DOH provided extra staff, known as locums, to the pharmacy to support implementation of the new records system, but during phase three the locum appointments ended, leaving the permanent pharmacy staff to reabsorb the full workload. Over the same period of time, the pharmacy manager changed twice. These disruptions affected the continuity of some operational improvements initiated by the project. They also underscored the widespread challenges posed by ongoing public sector human resource shortages and variations in staffing levels, along with attendant negative consequences for facility operations and care delivery [29-30]. Pharmacy staff also demonstrated varying degrees of participation at different points in the project. Factors in Clinic B Clinic B’s significant reduction in wait time and success in its operational improvements were bolstered by a variety of contextual enablers. Initial meetings between clinic leadership and the research team, along with subsequent student-run staff brainstorming sessions, were well attended and drew active participation from all levels of the facility. In the brainstorming sessions, doctors and nurses sat side by side with maintenance staff and clinic guards to generate and discuss ways to improve the clinic. Such an inclusive approach, with its less hierarchical form of joint idea generation and decision-making and its shared focus on learning, is in keeping with findings from other management initiatives in the health sector and beyond that attribute success to participation [55, 58-59]. At the end of phase two, the clinic benefitted from the previously scheduled booking system update. While the project did not give rise to the opportunity to update the appointment software, clinic 13 staff attributed their effectiveness in shaping its design and implementation to their experience with the action research collaboration. Clinic B also benefitted from strong, enthusiastic leaders who supported the full scope of facility-wide improvements, worked side by side with frontline staff throughout the project, and advocated for additional resources to help support change efforts. Amid these successes, Clinic B faced its own set of contextual barriers. Throughout the collaboration, staff turnover in various parts of the clinic required leadership to identify and train replacements. While the majority of staff members were engaged in the project, it is unsurprising that there were some who were less enthusiastic, occasionally demonstrating resistance to new ideas or data collection efforts. 4.3 Methodological implications and study limitations Our paper set out to explore shifts in wait times along with operational and other changes that took place during an action research collaboration at two sites. Iteration—which lies at the core of action research—shaped the present study’s methods for capturing wait times in each clinic, as linked cycles of planning, action, and evaluation led to alterations in our own methodology. For example, route slips were refined as the research team and clinic staff conducted initial tests and agreed on which fields to retain and which to eliminate. Experience with the paper slips paved the way for the development and eventual deployment of the electronic data collection tool used for the endline dataset. While there are obvious benefits to improving measurement, a potential drawback was the variation in collection methods across rounds. We confined the analysis presented in this paper to strictly comparable data: entry and exit timestamps that were captured at the same points in each collection round, along with visit types. In the course of our study we tracked multiple steps and categorical data for more than 3,200 patients, for which over 2,400 sets of records were used for analysis, yielding 70% coverage overall. No data collection process that impinges on staff and patients in the midst of a busy clinic could capture all records, particularly when volumes vary greatly by time of day and methods are manual, novel, or both. Due to variations in staff and research team experience with the different data collection methods and our stringent criteria for admissible data (any missing field, including for intermediate steps not reported here, resulted in exclusion), our dataset’s daily coverage levels varied (from around 49% to 82%). Yet as our comparison of patient mix data reveals, the overall proportions of visit types did not change, suggesting no bias in the variation in coverage due to changes in collection methods. The limited size of our sample set and lack of comparability in days of the week on which data was collected pose potential drawbacks but were necessitated by a variety of site-specific factors, such as scheduled training days, and personnel availability. Inevitably, clinic conditions differed by day (for instance, staffing levels fluctuated). 14 Finally, we suspect that our time horizon is too short to capture longer term results, both potentially negative and positive. More time to follow up may have yielded more positive results, as notable improvements in performance followed the last round of measurement. By necessity, the effects of some operational changes—such as providing refills for longer periods of time—would be apparent only weeks or months later, when patients would be spared more frequent return trips. Other operational changes simply took longer to implement. At the end of the final month of the study, Clinic A’s management reported an improvement in staff coordination, the re-uptake of rosters and five-minute morning meetings, and encouraging performance data. Clinic B continued to maintain and extend upon implemented changes. 5. Conclusion To understand how targeted, locally devised interventions could improve patient experience, we studied wait times and operational changes related to an action research study in two high-demand public primary care facilities. We compared mean entry-to-exit times before and after clinic staff worked with faculty-guided management graduate students to devise readily implementable solutions to each site’s operational challenges. In both cases, we found statistically significant reductions in patient waiting that saved patients a mean of 27 minutes in the pharmacy and 79 minutes in the clinic. The operational changes implemented at the two sites adjusted procedures, improved technology in use, tailored patient visits by type, and built new team capabilities. Contextual barriers included staff turnover, varying staff participation, mandated software changes, and resource constraints. Enablers included clinic leadership commitment, engagement of staff, experience with past improvement collaborations, advocacy, and a shared focus on appropriate and practical solutions. Our results underscore the potential value of enabling facilities to monitor their own operations. The data need not be complex: in the present study, basic time-stamp data on patient visits revealed the bottlenecks in each clinic, which in turn focused the team’s problem-solving efforts. Subsequently re-measuring wait times enabled staff to discern how wait times and bottlenecks were affected in the aftermath of operational changes. Such data is invaluable for anyone planning to make facility-level changes to improve patient experience, and to further their own learning about what works and what does not. To do this, there is a need for the right tools, such as Scanpoint-type electronic data collection, along with its enabling infrastructure. These are not presently widely available in sub-Saharan Africa. While developing a new tool was not a quick process—both software and hardware design required testing and iteration—the entire team found value in seeking better data collection and in the possibility that the resulting innovation could find use beyond the action research project. To benefit from such tools, however, also needed is an investment in clinic-level capabilities that would allow each facility to develop the capacity to collect data, ensure its quality, prepare datasets, and analyze the information. Support for doing so from within the healthcare system or other collaborators will likely prove important. 15 Our findings also reveal the promise of targeted, inclusive collaboration to trigger potentially beneficial change. Short term interventions are often rightly criticized for their lack of sustainability, inadequate contextualization, or overemphasis on technical interventions. Our experience reveals the potential value of brief, focused interventions when a jointly led, targeted collaboration is carefully designed to make the most of an external team that offers ideas, effort, methods, and analytical skills matched to the partner’s needs. Collaborative action in which change is jointly shaped by staff and outside partners may even benefit from a potentially unique attribute of short term projects—their ability to spur staff and leaders to approach longstanding problems with new perspectives, in a focused way necessitated by the brevity of the engagement. Pairing this urgency with an emphasis on sustainable changes is, we suggest, crucially important. Amid the complexity of operating a busy primary care facility and an array of structural and relational factors, the action research collaboration demonstrated that simple, low cost changes generated in situ can help address the vexing challenge of persistent long wait times. Both patients and staff can benefit from reduced crowding, better use of healthcare facilities, fewer complaints, and increased patient satisfaction. Such improvements could bring meaningful benefits to society in the form of increased adherence to treatment and improve the daily work experience of frontline staff. While we found wait time improvements to be sustained for months, and documented new implementation at the study’s end, it is clear that additional research is needed to assess their longer-term sustainability, track any subsequent side effects, test scalability, better measure outcomes, account for costs and benefits, and explore applicability to other domains. The research approach itself warrants a final word. Action research appears to be underutilized to explore the potential of frontline improvement efforts in low-resource settings, where widely noted challenges in healthcare delivery suggest the need for new configurations of tools and approaches—and a widespread sense that both action and evidence are urgently needed. Our observations could help others to learn from our experience as they work in this area. Action research methods allowed us to adapt, learn, and collaborate, while addressing practical challenges facing frontline facilities. Combining action research with systematic performance measurement allowed us to check that the changes generated by the collaboration were indeed improvements, a necessary first step in examining the value of action research. Along with this form of collaborative practical research, there is a need for the documentation and data collection methods used in the present paper to be developed further. We have only just begun to explore the potential of collaborative action interventions to change staff dynamics, operational efficiency, and patient outcomes. There is a potentially vast opportunity for frontline innovation to improve both staff and patient experience. 16 References 1. Miller CM, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Trop Med Int Health. 2010 June; 15(s1): 48-54. doi: 10.1111/j.1365-3156.2010.02514.x. 2. Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012 October 23;26(16): 2059-67. doi: 10.1097/QAD.0b013e3283578b9b. 3. Hardon A, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerritis T, et al. Hunger, waiting time and transport costs: Time to confront challenges to ART adherence in Africa. AIDS Care. 2007 May;19(5):658-65 4. Oche M, Adamu H. Determinants of patient waiting time in the general outpatient department of a tertiary health institution in north western Nigeria. Ann Med Health Sci Res. 2013 Oct-Dec;3(4): 588-92. doi: 10.4103/2141-9248.122123 5. Wagner G, Ryan G, Taylor S. Formative evaluation of antiretroviral therapy scale-up efficiency in subSaharan Africa. AIDS Patient Care STDS. 2007 Nov;21(11): 871-88. doi: 10.1089/apc.2007.0008. 6. Campbell C, Scott K, Madanhire C, Nyamukapa C, Gregson S. A ‘good hospital’: Nurse and patient perceptions of good clinical care for HIV-positive people on antiretroviral treatment in rural Zimbabwe—A mixed-methods qualitative study. Int J Nurs Stud. 2011 Feb;48(2): 175-83. 7. Umar I, Oche MO, Umar AS. Patient waiting time in a tertiary health institution in Northern Nigeria. J Public Health and Epidemiol. 2011 Feb;3(2): 78-82. 8. Singh H, Haqq ED, Mustapha N. Patients perception and satisfaction with health care professionals at primary care facilities in Trinidad and Tobago. Bull World Health Organ. 1999;77(4): 356-60. 9. McDonald HP, Garg AX, Haynes RB. Interventions to Enhance Patient Adherence to Medication Prescriptions: Scientific Review. JAMA. 2002;288(22): 2868-2879. doi:10.1001/jama.288.22.2868. 10. Camacho F, Anderson R, Safrit A, Jones AS, Hoffmann P. The relationship between patient’s perceived waiting time and office-based practice satisfaction. N C Med J. 2006 Nov-Dec;67(6): 409-13. 11. Peltzer K. Patient experiences and health system responsiveness in South Africa. BMC Health Serv Res. 2009 Jul; 14(9):117. doi:10.1186/1472-6963-9-117. 12. McCormack M. How Patients Use Online Reviews: IndustryView 2013. 2013 December 16. [cited 4 January 2015]. In: Software Advice [Internet]. Available: http://www.softwareadvice.com/medical/industryview/how-to-treat-patient-wait-time-woes/ 13. Anderson RT, Camacho FT, Balkrishnan R. Willing to wait? The influence of patient wait time on satisfaction with primary care. BMC Health Serv Res. 2007;7: 31. doi: 10.1186/1472-6963-7-31 14. Becker J, Dell A, Jenkins L, Sayed R. Reasons why patients with primary health care problems access a secondary hospital emergency centre. S Afr Med J. 2012; 102 (10). doi: 10.7196/samj.6059. 15. Fayyaz J, Khursheed M, Mir MU, Mehmood A. Odds for the patients who leave ED without being seen. BMC Emerg Med. 2013;13(1). doi:10.1186/1471-227X-13-1. 16. Guttman A, Schull MJ, Vermeulen MJ, Stukel TA. Association between waiting times and short term mortality and hospital admission after departure from emergency department: population based cohort study from Ontario, Canada. BMJ 2011; 342. doi:10.1136/bmj.d2983 17 17. Stahl JE, Drew MA, Leone D, Crowley RS. Measuring process change in primary care using real-time location systems: feasibility and the results of a natural experiment. Technol Health Care. 2011;19(6): 415-21. doi: 10.3233/THC-2011-0638. 18. Stahl JE, Holt JK, Gagliano NJ. Understanding performance and behavior of tightly coupled outpatient systems using RFID: initial experience. J Med Syst. 2011;35(3): 291-97. doi: 10.1007/s10916-0099365-7 19. Wanyenze RK, Wagner G, Alamo S, Amanyire G, Ouma J, Kwarisima D et al. Evaluation of the efficiency of patient flow at three Ugandan HIV clinics. AIDS Patient Care STDS. 2010 Jul;24(7): 4416. 20. Ndukwe HC, Omale S, Opanuga OO. Reducing queues in a Nigerian hospital pharmacy. African Journal of Pharmacy and Pharmacology. 2011 Aug;5(8): 1020-26. doi: 10.5897/AJPP11.015 21. Maluwa V, Andre J, Ndebele P, Chilemba E. Moral distress in nursing practice in Malawi. Nurs Ethics. 2012 March;19(2): 196-207. doi: 10.1177/0969733011414968. 22. Topp S, Chipukuma J, Giganti M, Mwango LK, Chiko LM, Tambatamba-Chapula B, et al. Strengthening health systems at facility-level: Feasibility of integrating antiretroviral therapy into primary health care services in Lusaka, Zambia. PloS One. July 2010;5(7): 1-11. doi: 10.1371/journal.pone.0011522. 23. Ameh N, Sabo B, Oyefabi MO. Application of queuing theory to patient satisfaction at a tertiary hospital in Nigeria. Niger Med J. 2013 Jan; 54(1): 64-67. doi: 10.4103/300-1652.108192. 24. Department of Health. White Paper for the transformation of the health system in South Africa. Government Gazette. Notice 667 of 1997, No 17910. Available: http://www.gov.za/sites/www.gov.za/files/17910_gen667_0.pdf. Accessed 23 Jan 2015 25. Chopra M, Lawn JE, Sanders D, Barron P, Abdool Karim SS, Bradshaw D, et al. Achieving the health Millennium Development Goals for South Africa: challenges and priorities. Lancet. 2009 Sep 19;374(9694): 1023-31. doi: 10.1016/S0140-6736(09)61122-3. 26. Dookie S, Singh S. Primary health services at district level in South Africa: a critique of the primary health care approach. BMC Family Practice 2012, 13: 67. doi: 10.1186/1471-2296-13-67 27. Naledi T, Barron P, Schneider H. Primary health care in SA since 1994 and implications of the new vision for PHC re-engineering. In: Padarath A, English R, editors. South African Health Review. 2011. Durban: Health Systems Trust; 2011: 17-28. 28. Whiteside A. South Africa’s key health challenges. Ann Am Acad Pol Soc Sci. 2014;652(1): 166-85. doi: 10.1177/0002716213508067 29. Mayosi BM, Benatar, SR. Health and health care in South Africa—20 years after Mandela. N Engl J Med. 2014 Oct 2;371(14): 1344-1353. doi: 10.1056/NEJMsr1405012 30. Kautzky K, Tollman SM. A perspective on primary health care in South Africa. In: Barron P, RomaReardon J, editors. South African Health Review 2008. Durban: Health Systems Trust; 2008: 17-30. 31. Motsoaledi A. Keynote speech, Inclusive Healthcare Innovation Summit, Bertha Centre for Social Innovation, University of Cape Town Graduate School of Business. Cape Town, South Africa. Jan 2014. 32. Department of Health, Western Cape Government. Western Cape Government Health Annual Report 2013/2014. Cape Town, South Africa: Western Cape Government; 2013. Accessed at http://www.westerncape.gov.za/sites/www.westerncape.gov.za/files/wcgdh14_annual_report_full_small .pdf 23 Jan 2015. 33. Western Cape Government Health. Healthcare 2030: The road to wellness. 2014 March. Available at: http://www.westerncape.gov.za/assets/departments/health/healthcare2030.pdf 18 34. Kahn T. Public Health: How Western Cape gets it right. Financial Mail. 4 Sept 2014. Available: http://www.financialmail.co.za/coverstory/2014/09/04/public-health-how-western-cape-gets-it-right. Accessed 23 Jan 2015. 35. Botha T. Western Cape Government Health Budget Vote 2014/15 by MEC Theuns Botha, Western Cape Provincial Parliament. 2014 March 20. Available at: http://www.health-e.org.za/wpcontent/uploads/2014/07/Western-Cape-Health-Budget-Speech-2014-15.pdf. Accessed 23 Jan 2015. 36. Scott V, Schaay N, Olckers P, Nqana, N, Lehmann U, Gilson L. Exploring the nature of governance at the level of implementation for health system strengthening: the DIALHS experience. Health Policy Plan. 2014;29: 59-70. doi:10.1093/heapol/czu073 37. Elloker S, Olckers P, Gilson L, Lehmann U. 2013. Crises, routines and innovations: the complexities and possibilities of sub-district management. In: Padarath A, English E, editors South African Health Review 2012/13. Durban: Health System Trust; 2013: 161-73. 38. Lehmann U, Gilson L. Action learning for health system governance: the reward and challenge of coproduction. Health Policy Plan. 2014:1-7. doi: 10.1093/heapol/czu097 39. Meyer J. Qualitative research in health care: using qualitative methods in health related action research. BMJ 2000;320: 178-181. doi:10.1136/bmj.320.7228.178 40. Bennett S, Agyepong IA, Sheikh K, Hanson K, Ssengooba F, Gilson L. Building the field of health policy and systems research: an agenda for action. PLoS Med. 2011 Aug 8;8(8): e1001081. doi:10.1371/journal.pmed.1001081 41. Gilson L, editor. Health Policy and Systems Research: A Methodology Reader. Alliance for Health Policy and Systems Research, World Health Organization. 2012: 443-467. 42. Burnes B. Kurt Lewin and the planned approach to change: A re-appraisal. Journal of Management Studies. 2004 Sept:41(6): 977-1002. 43. McNiff J. Action Research: Principles and Practice. 3rd ed. New York: Routledge; 2013. 44. Acosta S, Goltz HH. Transforming Practices: A Primer on Action Research. Health Promot Pract. 2014 Mar 31;15(4): 465-470. 45. Colebunders R, Bukenya T, Pakker N, Smith O, Boeynaems V, Waldron J et al. Assessment of the patient flow at the infectious diseases institute out-patient clinic, Kampala, Uganda. AIDS Care. 2007 Feb;19(2): 149-51. 46. Castelnuovo B; Babigumira J; Lamorde M; Muwanga A; Kambugu A; Colebunders R. Improvement of the patient flow in a large urban clinic with high HIV seroprevalence in Kampala, Uganda. Int J STD AIDS. 2009 Feb;20(2): 123-4. doi: 10.1258/ijsa.2008.008174. 47. Were MC, Sutherland JM, Bwana M, Ssali J, Emenyonu N, Tierneya WM. Patterns of care in two HIV continuity clinics in Uganda, Africa: A time-motion study. AIDS Care. 2008;12(6): 677-682. doi: 10.1080/09540120701687067. 48. Alamo ST, Wagner GJ, Ouma J, Sunday P, Marie L, Colebunders R, Wabwire-Mangen F. Strategies for optimizing clinic efficiency in a community-based antiretroviral treatment programme in Uganda. AIDS Behav. 2013 Jan;17(1): 274-83. 49. Deo S, Topp S, Garcia A, Soldner M, Yagci Sokat K, Chipukuma J, et al. Modeling the impact of integrating HIV and outpatient health services on patient waiting times in an urban health clinic in Zambia. PLoS ONE. 2012; 7(4): e35479. 50. Umar NA, Hajara MJ, Khalifa M. Reduction of client waiting time using task shifting in an antiretroviral clinic at specialist hospital Bauchi, Nigeria. J Public Health Africa. 2011;2(1). doi:10.4081/jphia.2011.e2 19 51. Ofili AN, Ofovwe CE. Patients’ assessment of efficiency of services at a teaching hospital in a developing country. Ann Afr Med. 2005;4(4): 150-153. 52. Kardas-Nelson M. African studies identify strategies for improving treatment adherence, retention in care. Aidsmap: Adherence. 7 Jan 2014. Available: http://www.aidsmap.com/African-studies-identify-strategies-for-improving-treatment-adherenceretention-in-care/page/2813706/. Accessed 23 Jan 2015. 53. Obua C, Kayiwa J, Waako P, Tomson G, Balidawa H, Chalker J, et al. Improving adherence to antiretroviral treatment in Uganda with a low-resource facility-based intervention. Glob Health Action. 2014;7. doi: 10.3402/gha.v7.24198 54. Gilson L, Daire J. Leadership and governance within the South African health system. In Padarath A, English R, editors. South African Health Review. 2011. Durban: Health Systems Trust; 2011: 69-80. 55. Gilson, L, Elloker, S, Olckers, P, Lehmann, U. Advancing the application of systems thinking in health: South African examples of a leadership of sensemaking for primary health care. Health Res Policy Syst. 2014; 12: 30. 56. Loewenson R, Flores W, Shukla A, Kagis M, Baba A, Ryklief A, et al. Raising the profile of participatory action research at the 2010 Global Symposium on Health Systems Research. MEDICC Rev. 2011; 13 (3): 35-38. 57. Franco LM, Marquez L: Effectiveness of collaborative improvement: evidence from 27 applications in 12 less-developed and middle-income countries. BMJ Qual Saf. 2011; 20(8): 658-665. doi: 10.1136/bmjqs.2010.044388. 58. Berman J, Nkabane EL, Malope S, Machai S, Jack B, Bicknell W. Developing a hospital quality improvement initiative in Lesotho. Int J Health Care Qual Assur. 2014;27(1): 15-24. 59. USAID Health Care Improvement Project. The Improvement Collaborative: An Approach to Rapidly Improve Health Care and Scale Up Quality Services. Published by the USAID Health Care Improvement Project. Bethesda, MD: University Research Co., LLC (URC); 2008. 20 i ur ic r t n a i r s uti n i a i ur ic r t n a i r s uti n i a i ur ic r t n a i r s uti n i a tri in a ic r t n a i r s uti n i a ictur ic r t n a t r ictur canp int stati n ti ictur ic r t n a t r ictur canp int p ti