* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energy
Thermodynamic system wikipedia , lookup
Heat capacity wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
R-value (insulation) wikipedia , lookup
Equipartition theorem wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Thermal radiation wikipedia , lookup
Temperature wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat transfer wikipedia , lookup
Adiabatic process wikipedia , lookup
Conservation of energy wikipedia , lookup
Thermal conduction wikipedia , lookup
State of matter wikipedia , lookup
Internal energy wikipedia , lookup
Heat transfer physics wikipedia , lookup
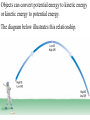
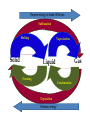
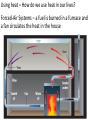
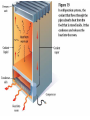
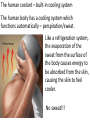
Unit 2 Matter, Energy, and Changes Energy Energy is the capacity to do work or produce heat. Energy • Forms: Radiant ex. sunlight Energy Kinetic energy carried by objects in motion (includes mechanical & thermal) Energy Potential due to position/stored energy (includes electrical & chemical) What is the Law of Conservation of Energy? Energy cannot be created nor destroyed….but…. Energy can be transformed from one type to another. In this diagram, electrical energy is converted into thermal (heat) energy and light energy. There are many energy transformations in an automobile. The explosion of the gasoline involves the release of chemical energy. The chemical energy causes the pistons in the engine to move (mechanical energy) The moving pistons affect many moving parts including a generator which produces electrical energy which keeps the battery charged. The moving pistons and other moving parts also produce heat energy. Objects can convert potential energy to kinetic energy or kinetic energy to potential energy. The diagram below illustrates this relationship. Matter What holds one molecule or atom to another? Intermolecular Forces (forces between neighboring particles) Intermolecular Forces • There are different kinds of intermolecular forces. • Some are very weak and break easily. • But many of them together make a strong network. Physical State of Matter State of matter at room temperature depends on strength of intermolecular (IM) forces • For example, a substance with strong IM forces will be a solid while a substance with very weak IM forces will be a gas • To understand why intermolecular forces are important, we must first talk about molecule movement and kinetic energy • The Kinetic Molecular theory helps explain why a solid has certain behaviors that are different than gases or liquids. Kinetic Theory and states of Matter • Kinetic theory says that molecules are in constant motion. • Perfume molecules moving across the room are evidence of this The Kinetic Theory of Gases • Kinetic Theory helps to explain why a gas behaves as it does. • It also helps us understand the changes in physical states of matter The Kinetic Theory of Gases Makes three descriptions of gas particles 1. A gas is composed of particles, molecules, • • or atoms Considered to be hard spheres far enough apart that we can ignore their volume. Between the molecules is empty space. 2. The particles are in constant random motion. • Move in straight lines until they bounce off each other or the walls. 3. All collisions are perfectly elastic • The molecules don’t travel very far without hitting each other so they move in random directions. States: Gas no definite shape or volume particles randomly scattered particles moving quickly in constant motion low density highly compressible rapid diffusion high expansion on heating States:Liquid no set shape definite volume particles somewhat organized particles free to move slightly compressible high density slow diffusion low expansion on heating Properties of Liquids Viscosity – friction or resistance to motion, increases as temperature decreases Surface tension – molecules at the surface experience imbalanced attractive forces • Both gasses and liquids are fluids. –This is because of weak intermolecular forces. –The molecules can slide easily over each other. States:Solid • has particular shape • definite volume • particles very organized and close together • particles move only very slightly • low compressibility • high density • slow diffusion • low expansion on heating • Intermolecular forces are strong • Molecules move - can only vibrate and revolve in place (they cannot flow past each other) • Melting point, MP, is the temperature where a solid turns into a liquid (MP is the same as the freezing point) • When heated, the particles vibrate more rapidly until they shake themselves free of each other. • As they are heated the temperature doesn’t change. – The energy goes into breaking bonds, not increasing motion. – Move differently, not faster. Basic Types of Solids Crystalline solids – Amorphous solids - Crystalline Solids • are made of atoms arranged in highly ordered, patterns called unit cells (regular repeating three dimensional arrangement of atoms in a solid) • Most solids are crystals • Break at certain angles – Examples: table salt, table sugar, emeralds Amorphous solids • appear solid, but are more of a super-cooled liquid, have high viscosity, gradually soften as temperature increases • Rigid but lacking orderly internal structure • Do not melt - just gradually get softer as temperature increases • Shatter at random angles • Examples: glass and plastics Properties of Solids Some solids are good conductors due to particles being in contact with each other and passing the energy from one to another Matter & Changes Changes of State Energy and change of state… To change states, particles must overcome the attractive forces holding them together (the number of particles does not change) Changes of State Vaporization Liquid changes to a gas, also called evaporation, requires energy input to break the intermolecular forces Changes of State Vaporization Liquid changes to a gas, also called evaporation, requires energy input to break the intermolecular forces Rapidly moving particle near surface of liquid gains enough energy to escape attractive forces of other particles Changes of State Vaporization Liquid changes to a gas, also called evaporation, requires energy input to break the intermolecular forces Rapidly moving particle near surface of liquid gains enough energy to escape attractive forces of other particles Volatile liquid – one that readily evaporates Changes of State Is vaporization the same as evaporation? • Not quite… – Vaporization - the change from a liquid to a gas below its boiling point – Evaporation - vaporization of an uncontained liquid (no lid on the bottle) Changes of State More on Evaporation… • Molecules at the surface break away and become gas • Only those with enough KE escape • Evaporation is a cooling process. • It requires energy Changes of State Boiling • Making bubbles of gas • Forces liquid level to rise • Must push against air pressure on the liquid Changes of State Boiling • A liquid boils when the vapor pressure = the external pressure (temperature is called the boiling point) • Normal Boiling point is the temperature a substance boils at 1 atm pressure. – The normal boiling point of water is 100o C • The temperature of a liquid can never rise above it’s boiling point • Energy goes into breaking forces, not moving faster Changes of State Changing the Boiling Point… • Lower the pressure (like going up into the mountains) – Lower external pressure requires lower vapor pressure – Easier to make bubbles – Lower vapor pressure means lower boiling point. – Food cooks slower Changes of State Changing the Boiling Point… • Raise the external pressure (like using a pressure cooker) – Raises the vapor pressure needed – Harder to make bubbles – Raises the boiling point – Food cooks faster Changes of State Boiling point differences… Different substances boil at different temperatures because they have • Different intermolecular forces – Weak forces → lower boiling point • Different vapor pressures – Low vapor pressure → high boiling point Changes of State Condensation • Change from gas to liquid • Molecules stick together • Releases energy Changes of State Freezing (Solidification) Particles get closer together and more organized than in the liquid state, releases energy Changes of State Melting (Liquification) Particles become less organized and farther apart, requires energy input to break the intermolecular forces Changes of State Melting Melting point - temperature at which solid and liquid form of substance exist in equilibrium, also called freezing point Changes of State Sublimation Solid changes directly to a gas, requires energy input to break the intermolecular forces Changes of State Deposition Gas changes directly to a solid, releases energy Require energy to break IM forces Sublimation Melting Solid Vaporization Gas Liquid Freezing Condensation Deposition Release energy Changes of State Heating curves • Describe changes of state of matter, plot of sample temperature as a function of time Changes of State Changes of State Phase diagrams Relates states of matter to temperature and pressure Changes of State Changes of State Temperature and Phase Change • The temperature doesn’t change during a phase change. • If you have a mixture of ice and water, the temperature is 0ºC • At 1 atm, boiling water is 100ºC • You can’t get the temperature higher until it finishes boiling Energy Measuring: • calories (cal) – amount of heat needed to raise temperature of 1 g H2O by 1 C 1 cal = 1 g × 1 C •Food energy is in Calories, 1 Cal = 1 kcal Energy Measuring: • SI unit is Joule (J), 1 J is about the energy to lift a medium-sized apple 1 meter from ground 1 cal = 4.184 J Energy Law of Conservation of Energy: in any process, energy is neither created nor destroyed Temperature What is temperature? • comparison of how hot or cold an object is with some standard • measure of the average kinetic energy of the particles in a sample of matter. • The higher the average KE of the molecules the faster the molecules are moving => Higher temperature Temperature Units for Temperature • Fahrenheit • Celsius • Kelvin (SI units) Temperature Units for Temperature • Kelvin (SI units) Why don’t we use a degree mark with Kelvin temperatures? Lowest temperature reading in Kelvins is called Absolute zero At this temperature KE is zero. Movement stops Temperature Units for Temperature A quick look at the three scales. 0 C = 273 K = 32 F 100 C = 373 K = 212 F Temperature Converting Fahrenheit/Celsius • C = 5/9 × (F – 32) Temperature • Convert 98.6 F to C Temperature • Convert 25 C to F Temperature Converting Kelvin/Celsius • C = K – 273 Temperature • Convert 399 K to C Temperature • Convert 25 C to K What is Thermal Energy? Particles of matter are in constant motion. This motion relates directly to the state of matter of the object (solids, liquids, or gases). Temperature affects how fast these particles move. The higher the temperature the faster the particles move. Moving particles possess kinetic energy. Temperature is defined at the average kinetic energy of the particles of an object. Thermal Energy is the sum total of all of the energy of the particles of an object. Thermal energy and temperature are related though DIFFERENT. Temperature is the average kinetic energy of the particles of an object. Thermal energy is the total amount of energy of the particles of an object. A bathtub full of water at 100oF has more thermal energy than a thimble of water at 100oF. The temperature is the same but the total amount of energy is different. The bathtub has more energy. What is heat? Heat is thermal energy that flows from something at a higher temperature to something at a lower temperature. What is specific heat? Specific heat is a property of matter which determines how readily a material absorbs heat and changes temperature. Specific Heat is defined as the amount of heat energy needed to raise the temperature of 1 kg of a substance by 1 Co or 1 K. Notice that water has a very high specific heat, whereas iron has a low specific heat. Water requires a lot of heat energy to raise its temperature. What is heat? Heat is thermal energy that flows from something at a higher temperature to something at a lower temperature. What is specific heat? Specific heat is a property of matter which determines how readily a material is to absorb heat and change temperature. Specific Heat is defined as the amount of heat energy needed to raise the temperature of 1 kg of a substance by 1 oC or 1 K. Transferring Thermal Energy (YOU MUST KNOW THIS!!!!!) Conduction – direct contact Convection – through a fluid Radiation – by electromagnetic waves What is heat transfer by conduction? •Heat transfers as particles of an object increase their collisions as they are heated. These collisions transfer the heat energy through the object by colliding with adjacent particles. What is heat transfer by convection? •Heat is transferred through a substance through currents. This occurs in fluids (liquids AND gases) •Convection currents are caused by heating of a liquid or gas, the liquid or gas rises, then cools and falls. This occurs in the mantle of the earth. •And in the atmosphere. •Most of our weather patterns are the result of convection currents in the atmosphere. What is heat transfer by radiation? •Radiation is heat transfer by electromagnetic waves. These waves may pass through all states of matter and also through NO matter – such as the vacuum of space. •This energy is often called radiant energy. •Radiant energy from the sun travels through the vacuum of space until it reaches the earth. How is heat flow controlled? Insulators – a material which does not allow heat to pass through it easily. Some animals have good insulation to survive severe winters. Buildings and houses are insulated so that heat does not pass out of them in winter or into them in summer. What are some other uses of insulation? Some animals have good insulation to survive severe winters. Using heat – How do we use heat in our lives? Forced-Air Systems – a fuel is burned in a furnace and a fan circulates the heat in the house Radiator Systems – closed metal containers that contain hot water or steam. The thermal heat is transferred to the air and circulated by convection currents. Electric radiators – heat metal coils which transfer the thermal heat to the surrounding air. Refrigerators, air conditioners, and heat pumps – how do they work? A coolant is circulated through pipes by a compressor. When the pipes get small and narrow the coolant compresses and changes to a liquid giving off heat energy. When the pipes get larger, the coolant changes from a liquid to a gas (evaporates) causing the area to absorb heat and get cooler The human coolant – built-in cooling system The human body has a cooling system which functions automatically – perspiration/sweat. Like a refrigeration system, the evaporation of the sweat from the surface of the body causes energy to be absorbed from the skin, causing the skin to feel cooler. No sweat!!!