* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download the taxonomic status of mimulus sookensis
Survey
Document related concepts
Transcript
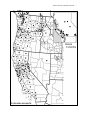
Nesom, G.L. 2013. The taxonomic status of Mimulus sookensis (Phrymaceae) and comments on related aspects of biology in species of Erythranthe. Phytoneuron 2013-69: 1–18. Published 26 September 2013. ISSN 2153 733X THE TAXONOMIC STATUS OF MIMULUS SOOKENSIS (PHRYMACEAE) AND COMMENTS ON RELATED ASPECTS OF BIOLOGY IN SPECIES OF ERYTHRANTHE GUY L. NESOM 2925 Hartwood Drive Fort Worth, Texas 76109 [email protected] ABSTRACT Mimulus sookensis Benedict et al. (2012) is a tetraploid from the Vancouver Island area, western Oregon, and northwestern California hypothesized in molecular studies to be of hybrid origin between diploid Erythranthe [Mimulus] nasuta and diploid Erythranthe [Mimulus] guttata. The putative non-nasutus parent in these studies apparently is more accurately identified as Erythranthe [Mimulus] microphylla. Molecular evidence indicates that M. sookensis had two independent origins (disjunct northern and southern population systems) or perhaps as many, at least, as 11 independent origins. In the study that identified two origins, molecular evidence clusters the non-nasutus parent of the northern tetraploids among populations sampled from central California counties, but the nonnasutus parent of the southern tetraploids was not identified. Mimulus sookensis and typical E. nasuta often can be distinguished by slight differences in sizes when growing side-by-side, but size ranges of plants, flowers, and fruits are completely overlapping when the populational perspective is broadened, and the tetraploid is otherwise similar to the diploid in every morphological respect. It is impossible to distinguish them in the herbarium. In contrast, experimental hybrids and naturally occurring nasuta-microphylla hybrids apparently of contemporary origin are intermediate in morphology. Other examples of hybridization and introgression between Erythranthe nasuta and E. microphylla/E. guttata are reviewed. The origin and status of the hexaploid hybrid Mimulus peregrinus are reviewed and two associated nomenclatural combinations are made: Erythranthe peregrina (Vallejo-Marín) Nesom, comb. nov., and Erythranthe × robertsii (Silverside) Nesom, comb. nov. KEY WORDS: Mimulus sookensis, Erythranthe sect. Simiola, allopolyploid species, independent evolutionary origin, recurrent hybrid, asymmetric introgression, Mimulus peregrinus Plants hypothesized to represent an undescribed species on Vancouver Island were informally described and named by Benedict (1993), who considered the possibility that the entity originated as a tetraploid hybrid between two diploids, autogamous Mimulus nasutus Greene and allogamous M. guttatus Fisch. ex DC. She also found similar tetraploids in southwestern Oregon and more were later documented from other western Oregon localities as well as northern California (Sweigart et al. 2008). The taxon was later validly named as Mimulus sookensis by Benedict et al. (2012). Mimulus sookensis is confirmed as a tetraploid by flow cytometry and chromosome counts, and allozyme data indicated to Benedict that most individuals are fixed heterozygotes, this corroborated by subsequent molecular-genetic studies (Sweigart et al. 2003; Benedict et al. 2012; Modliszewski & Willis 2012). Following Benedict's investigation, molecular studies have confirmed the hybrid nature of the tetraploids and crossing experiments show that they are reproductively isolated from their putative diploid parents –– progeny of interploid backcrosses produce less than 2% viable seeds and plants from those few are infertile (Benedict 1993; Sweigart et al. 2008). Triploids have not been encountered in the field. Nesom: Status of Erythranthe sookensis 2 The first suggestion that Mimulus sookensis was an distinctive entity was from failed crosses between it and M. microphyllus in experiments by Tony Griffiths and Fred Ganders (F. Ganders pers. comm., 2013) aimed at investigating the genetics of flower size. They had assumed the smallflowered plants were M. nasutus, which was then known to cross with its relative. Ganders later suggested the problem in taxonomy as a thesis project to Benedict. Remarkably, the first allusion to M. sookensis in literature appeared in 1983 (Griffiths & Ganders 1983, p. 167), in comments on M. guttatus. "A possible example of an ecotype is a small form of yellow monkey flower, called variety depauperatus (Figure 5.2). Hitchcock and Cronquist's Flora notes that it occurs in less wet habitats than the more robust variety guttatus. However, we have observed the small form on Nanoose Hill and Mill Hill near Victoria growing together with the large. The small form flowers much earlier than most of the large plants on Nanoose Hill. We have tried crossing the two varieties but with no luck, so they could be different species." [The discussion apparently was referring to the tetraploid = M. sookensis (the 'small' form) and diploid = M. microphyllus (the 'large, robust' form.)] The distribution of Mimulus sookensis appears to be disjunctly apportioned between two regions (Fig. 3), in the limited sampling to date. The northern segment includes the Gulf Islands (Saltspring, Mayne, Galiano, Denman, Lasqueti, and Pender islands) and the southern end of Vancouver Island, British Columbia, and the San Juan Islands of Washington. The southern segment includes western Oregon –– along areas of the Willamette River (Lane Co.), Umpqua River (Douglas Co.), and Rogue River (Josephine Co.) –– and northwestern California (Mendocino Co.). As noted below, molecular data indicate that evolutionary origins of the two geographic segments are independent and that multiple origins may have occurred even within the two segments. In a formal taxonomic context, the name for the tetraploids would be positioned in the genus Erythranthe Spach, one of the segregates established by Barker et al. (2012) among the species of Mimulus sensu lato. It may be, however, that an earlier name exists for the tetraploids –– either M. subreniformis Greene or M. puncticalyx Gandoger (see localities in Fig. 3 and formal citations below), both of which were treated by Nesom (2012) as synonyms of M. nasutus. These names were considered by Benedict et al. (2012) but given the apparent impossibility of distinguishing tetraploids from diploids based on morphology, a chromosome count would be necessary for identification. Mimulus sookensis Benedict, Modliszewski, Sweigart, Martin, Ganders, & Willis, Madroño 59: 34. 2012. TYPE: CANADA. British Columbia. On a SW-facing, open, wet hillside in Sooke Potholes Provincial Park beside the Sooke River, elev. 75 m, 48º 24' N, 123º 43' W, 1 May 1991, B.G. Benedict 28 (holotype: UBC V207976 digital image!, see UBC online type database; Fig. 1). Possible earlier names for the tetraploid: Mimulus subreniformis Greene, Erythea 3: 67. 1895. LECTOTYPE (Nesom 2012, p. 62): USA. California. Shasta Co.: Burney Falls, 30 May 1894, M.S. Baker and F. Nutting s.n. (ND-Greene 46422! photo-PH!, photo-UT!; isolectotypes: ND-Greene!, UC! Fig. 2). Benedict et al. apparently saw the UC specimen, citing it as "holotype" and noting that it "Appears to be a diminuitive variant of M. nasutus, but without anthocyanin spotting on corolla." Mimulus puncticalyx Gandoger, Bull. Soc. Bot. Fr. 66: 219. 1919. TYPE: USA. Washington. [Klickitat Co.:] Ad Bingen, no date, W.N. Suksdorf 2775 (holotype: LY?; isotypes: ORE, PH-2 sheets!, WS digital image! photo-PH!, WTU 2 sheets). Benedict et al. apparently saw the ORE isotype, noting "Leaves tiny, upper tooth hardly more prominent than others; only a single specimen was examined in the naming." Mimulus puberulus Gandoger, Bull. Soc. Bot. Fr. 66: 219. 1919, nom. invalid. [non Greene ex Rydb. 1906]. TYPE: USA. Washington. Klickitat Co.: Bingen, riverbank, 17 Apr 1905, W.N. Suksdorf 5016 (holotype: LY?; isotypes: MO!, US digital image!, WS photo-PH!, WTU). The WS sheet was photographed for the PH collection at the "home of W.N. Suksdorf." Nesom: Status of Erythranthe sookensis Figure 1. Mimulus sookensis Benedict et al. Holotype, UBC. 3 Nesom: Status of Erythranthe sookensis Figure 2. Mimulus subreniformis Greene. Isotype, UC. See text and Map 1. Diploid or tetraploid? 4 Nesom: Status of Erythranthe sookensis 5 In the comments below, plants mostly are identified as Mimulus rather than Erythranthe, not because of an ambivalence regarding their taxonomic placement but instead for ease of comparison with the literature under consideration. Distinction from Mimulus nasutus Mimulus sookensis is described as a cryptic species "exceedingly similar in floral morphology to M. nasutus. ... All characters overlap to a degree with M. nasutus, but under favorable growth conditions, [some] structures tend to be more reduced in M. sookensis" (Benedict et al. 2012, p. 10). "Under most growing conditions, [M. nasutus, M. gutattus, and the tetraploid] can be distinguished morphologically. However, when collected from dry habitats the two small-flowered species are difficult to separate" (Benedict 1993, p. ii). Modliszewski and Willis (p. 5282) also noted that M. sookensis "is strikingly similar to M. nasutus and difficult to distinguish in the field." It also is difficult to distinguish in the herbarium –– in a taxonomic study of Erythranthe sect. Simiola (Nesom 2012), I identified and mapped all tetraploid specimens that might have come before me, if any did, as Erythranthe (Mimulus) nasuta (see Fig. 1). Even now, upon encountering the holotype of M. sookensis in a herbarium collection, without knowledge of its chromosome number, I would surely identify it as E. nasuta. In the current study, I have identified and annotated all nasutus-like collections simply as E. nasuta. Contrasts between Mimulus sookensis and M. nasutus noted by Benedict (1993) and Benedict et al. (2012) are summarized here as a key couplet. 1. Stems 3–25 cm high, less than 1 mm wide, often less sharply angled and winged; leaves 0.5–3 x 0.5–2.5 mm; stipes 0–1 mm long; pedicels 3–22 mm long; calyces 5–13 mm long, more frequently with anthocyanic red spotting; corolla tube-throat narrowly funnel-shaped; maturing ovary or fruit usually 2.5–3.5 mm longer than the calyx .......................................................................................................................... Mimulus sookensis 1. Stems 5–50 cm high, less than 4 mm wide, often more sharply angled and winged; leaves 0.5–10 x 0.5–7.5 mm; stipes 0.5–2 mm long; pedicels 4–26 mm long; calyces 6–16.5 mm long, less frequently with anthocyanic red spotting; corolla tube-throat nearly cylindrical; maturing ovary or fruit usually equal or up to 6 mm shorter than the calyx ............................................................................................................................ Mimulus nasutus Benedict et al. also noted that a red blotch on the lower corolla lip is characteristic of M. nasutus but does not appear in M. sookensis. From personal observation, however, the red blotch is not a constant feature of M. nasutus –– it is absent in otherwise typical populations from various parts of its range. Examination of vouchers and other specimens at UBC (where Bennett and Modliszewski worked and studied) shows that they identified (by annotation) all but two nasutus-like collections from Vancouver Island as Mimulus sookensis –– Benedict 7 (Nanoose Hill) and 8 (Gabriola Island) are identified as M. nasutus. Modliszewski in 2012 annotated as M. sookensis only those collections that had previously been so identified by Benedict. Among the other 15 UBC collections of E. nasuta (variously identified originally as M. alsinoides, M. guttatus, and M. nasutus), only one of them was annotated as M. nasutus by Benedict, none by Modliszewski, except for Benedict s.n. from Tuolumne Co., California, which was correctly identified initially by the collector. It appears at UBC that neither Benedict nor Modliszewski attempted to apply their criteria for distinguishing M. sookensis from M. nasutus to a wider range of collections, or else they felt restrained by uncertainty from annotating. Parentage Benedict (1993) was circumspect in discussing potential parentage of the tetraploids. She noted that "... it is not clear which living species if any in the Mimulus guttatus complex are the progenitors of [the tetraploid]. I will therefore compare [the tetraploid] to the two annual members of Nesom: Status of Erythranthe sookensis Figure 3. Distribution of Erythranthe nasuta and known populations of Mimulus sookensis. Mimulus puncticalyx (type from Klickitat Co., Washington) and M. subreniformis (type from Shasta Co., California) are possible earlier names for the tetraploid M. sookensis. 6 Nesom: Status of Erythranthe sookensis Figure 4. Distribution of Erythranthe microphylla. British Columbia distribution continues with inset. 7 Nesom: Status of Erythranthe sookensis 8 the M. guttatus complex with which it can grow sympatrically [M. nasutus and M. guttatus], particularly M. nasutus with which it shares its breeding behavior, certain life history traits and a similar morphology. It should be noted also that M. platycalyx was found growing in Oregon within a mile of and on a very similar site to the [tetraploid] population 91-17 [in Douglas Co.]" (p. 108). Perhaps underlying Benedict's reluctance to specify parents was her observation of Mimulus nasutus-guttatus hybrids of intermediate morphology (unlike M. sookensis) at two localities –– one in Calaveras Co., California, and one on Nanoose Hill, Vancouver Island. On Nanoose Hill, she observed four entities growing in close sympatry: M. nasutus, M. guttatus, M. nasutus-guttatus hybrids, and M. sookensis (e.g., see Benedict, pp. 38 and 99 for sympatry of the three species, p. 26 for reference to the nasutus-guttatus hybrids). Further examples of similar hybrids are noted below. The name Mimulus guttatus has commonly been over-broadly applied to plants that are more precisely identified as M. microphyllus, M. grandis Greene, M. guttatus in the strict sense, and sometimes others as well (Nesom 2012). Molecular-genetic studies of Mimulus (as well as collectors of specimens) have commonly identified this conglomeration of entities simply as M. guttatus. Reference to M. guttatus as a putative parent of M. sookensis, by Benedict and others, apparently is more accurate as M. microphyllus (= Erythranthe microphylla (Benth.) Nesom; Fig. 4; Nesom 2013a, 2013b). Mimulus microphyllus often has been termed the "inland annual race of Mimulus guttatus" (e.g., Lowry et al. 2008), and Benedict described putatively parental M. guttatus as annual, a feature characteristic of M. microphyllus but not M. guttatus in the strict sense, which is rhizomatous or stoloniferous and usually characterized as perennial (Nesom 2012, 2013a). Subsequent to Benedict's original study, "Mimulus guttatus" has consistently been advanced as one of the parents of M. sookensis, but evidence for its parental contribution remains strongly circumstantial, even when considering it to be M. microphyllus. What is known is this. In each of the tetraploids, two haplotypes occur at each of the nuclear genes mCYCA and mAP3 (Sweigart et al. 2008). The mCYCA haplotypes characteristic of the Vancouver region and the California-Oregon region both share near-identity with sequences from M. nasutus. The "non-nasutus" mCYCA haplotype characteristic of the Vancouver region clusters within a group of "M. guttatus" populations from six central California counties (Merced, Mono, Placer, San Benito, Stanislaus, and Tuolumne). The "non-nasutus" mCYCA haplotype characteristic of the California-Oregon region is not matched in any members of the M. guttatus complex sampled by Sweigart et al. The rationale for considering M. guttatus a parent is this, as provided by Sweigart et al. (p. 2097): "All tetraploids carry a haplotype at each locus that clusters unambiguously with sequences from Mimulus nasutus, implicating the diploid species as an ancestor. However, assigning ancestry for the second, divergent haplotype at each locus is not as straightforward. Although these haplotypes share substantial similarity with sequences from M. guttatus, we did not find any exact matches to haplotypes sampled from that diploid species. This result is perhaps not surprising given high rates of recombination and extensive polymorphism within M. guttatus (Sweigart & Willis 2003). Nevertheless, M. guttatus seems the most likely candidate to have given rise to the allotetraploid. Relative to other members of the complex, M. guttatus is the most widely distributed, and its range overlaps extensively with that of the widespread M. nasutus. Indeed, all other species of the M. guttatus complex have much more localized distributions, with many restricted to central California ... . Therefore, we argue that these Mimulus polyploids are allotetraploids, likely formed by hybridization between M. nasutus and M. guttatus. Nesom: Status of Erythranthe sookensis 9 And this (p. 2093). "For both genes, (nuclear genes mCYCA and mAP3) all tetraploids carry one haplotype that shares near identity with sequences from M. nasutus. In addition, all tetraploids carry a second, divergent haplotype that cannot be resolved from M. guttatus or other members of the complex. We interpret this pattern as evidence that these Mimulus polyploids are allotetraploids formed by interspecific hybridization between M. nasutus and M. guttatus ... . " A statement by Modliszewski and Willis (p. 5291) is similar. "Because M. nasutus is typically represented by a single or small number of haplotypes (Sweigart & Willis 2003; Sweigart et al. 2008; this study), one of the copies of M. sookensis tends to group very closely with M. nasutus, while the other copy appears more similar to M. guttatus. In most cases, the tight clustering of one of the homeologs of M. sookensis with M. nasutus and the similarity of the second homolog to M. guttatus allows the parentage of each homeolog to be identified." In sum, the actual second parentage ("non-nasutus") of Mimulus sookensis has not been identified in the more secure sense that M. nasutus has been. Attribution of parentage to M. microphyllus rests on (1) its proximity (as potential parent) to the tetraploids and (2) a similarity of the non-nasutus mCYCA haplotype of the Vancouver region tetraploids to a subgroup of central California plants (morphology unknown but all annual in duration, fide Seed Collections 2009 and previously published studies using the same populations) over-broadly identified simply as Mimulus guttatus. The non-nasutus parent of the California-Oregon segment was not identified in any sense. Neither Sweigart et al. (2003) nor Sweigart and Willis (2008) included a sample of Mimulus guttatus (in broad or narrow sense) from the Vancouver region. Such samples were included by Modliszewski and Willis (2012), but they did not examine mCYCA locus, thus an extended neighborjoining analysis could not make the results comparable to the earlier 2-gene studies that show population clustering. Characterization of molecular variation within M. microphyllus and of molecular profiles of other species of the Microphylla group perhaps will provide insight into the identity of the second parent of the California-Oregon region tetraploids. Accurate identification of the California populations (vouchers needed) with which the Vancouver tetraploids cluster may be critical and central to the interpretation of identity. From the observations that independently derived southern and northern population segments of the tetraploids are closely similar in morphology and interfertile (Benedict 1993; Modliszewski & Willis 2012), it seems reasonable to expect that non-nasutus parentage of both segments is similar –– perhaps involving ecotypic variants of a single species or else distinct but closely related species. There is no evidence at hand to eliminate the unconsidered hypothesis that the California-Oregon non-nasutus ancestor is extinct. Benedict's Mimulus platycalyx In the recent taxonomic review of Erythranthe sect. Simiola (Nesom 2012), Mimulus platycalyx Pennell is placed as a synonym of Erythranthe microphylla, but Benedict distinguished the former from "annual Mimulus guttatus" (= E. guttata) and noted that "in addition to recognizing [M. sookensis], M. platycalyx and M. nasutus as distinct species, taxonomic treatments should also recognize that the latter two are both capable of hybridization with M. guttatus in the field." She included M. platycalyx from Douglas Co., Oregon, in experimental crosses, noting (p. 26) that "all eight crosses attempted between M. guttatus and M. platycalyx were successful as were two out of the three crosses attempted between M. nasutus and M. platycalyx. Nesom: Status of Erythranthe sookensis 10 Benedict's refererences to Mimulus platycalyx and its inclusion in her hybridization studies suggest that she was reluctant to eliminate it from consideration in the potential parentage of the tetraploid. In fact, she observed (p. 113) that "If it is assumed that M. nasutus is one of the progenitors of [M. sookensis] then the decrease in calyx size in [M. sookensis] as compared to M. nasutus could be explained if a short-calyx species such as M. platycalyx was the other parent." In the later review and formal taxonomic proposal by Benedict et al. (2012), M. platycalyx was not mentioned as a M. guttatus relative or even among numerous synonyms. The two UBC specimens identified as Mimulus platycalyx are growth chamber-grown plants from seeds collected by Benedict in Douglas Co., Oregon (W-facing bank near Kellogg Springs Camp turnoff near Hwy 138 in the Umpqua River Valley, 13 Apr 1991, Benedict 25 –– UBC V207993a and UBC V207993b, see photos on the UBC database). It is not clear if either of these plants was used in the hybridization studies, but it seems reasonable to assume that the study parent(s) was diploid. Both specimens of Benedict 25 are essentially referable to Erythranthe microphylla, but the two are widely divergent in morphology, apparently as segregants of genetically heterogeneous parents, neither plant close to the mode of characteristic morphology for E. microphylla. F1 and F2 hybrids Martin and Willis (2007) noted that F1 hybrids between Mimulus nasutus and M. microphyllus are "intermediate for a variety of floral characters that distinguish the two species," and Benedict (1993) made similar observations about nasutus-microphyllus F1 hybrids. Fishman et al. (2002) found that F1 and F2 hybrids between M. nasutus and the much-used "Iron Mountain" population (Linn Co., west-central Oregon) of M. microphyllus have chasmogamous flowers "that are much more similar in size to M. [microphyllus], due to dominance of the M. [microphyllus] floral genes." They noted that "Although the F2 population showed an increase in variance relative to the parental and F1 classes, both parental extremes were not reconstituted. This suggests the segregation of many genes of small to moderate effect on floral characters" (p. 2142). Fishman et al. did not specify the ploidy level of the hybrids but apparently had no reason to suspect that they were tetraploid. The genetic biology of the sookensis tetraploids is different from that of the experimental nasutus-microphyllus hybrids. Modliszewski and Willis observed (p. 5295) that F2 hybrids of Mimulus sookensis crosses (between plants hypothesized to be of independent origin) do not segregate for flower size –– indicating that nasutus and microphyllus homeologs consistently pair only with each other and that lack of recombination underlies the lack of variability, in contrast to meiosis in the recently constituted, probably diploid, hybrids. Independent origins The northern and southern population segments of Mimulus sookensis are indicated by molecular data to have independent origins (Benedict 1993; Sweigart et al. 2008; and as directly implied in the comments immediately above). Two geographic/genetic subgroups among the M. guttatus-like tetraploid mCYCA haplotypes are evident in the phylogenetic analysis by Sweigart et al. (see comments above); in contrast, there is little variation among M. guttatus-like haplotypes at the mAP3 locus. Based on sequence data from six nuclear loci and previously published results in mCYCA and mAP3, Modliszewski and Willis (2012) observed that each of 11 of the 16 populations in their study possibly had a unique origin, but they acknowledged that the estimate of independent origins may be upwardly biased because of hybridization within Mimulus sookensis (p. 5292) and because they found no significant reductions in pollen viability in hybrid progeny of M. sookensis x M. sookensis (p. 5294). High pollen viability presumably would indicate regular chromosome pairing and meiotic Nesom: Status of Erythranthe sookensis 11 behavior, and photos of meiosis in M. sookensis (Benedict et al. 2012, Fig. 2) do not show any obvious irregularities. Presumably without the "upward bias," the number of unique origins might be reduced to two, as earlier hypothesized by Sweigart et al. Genetic variation within Mimulus guttatus in the broad sense Current concepts of wide genetic diversity among populations in Mimulus guttatus appear to be centrally derived from studies by Sweigart et al. (2003) and Sweigart and Willis (2008), which show neighbor-joining trees of many and geographically diverse populations, based on mCYCA and mAP3 sequences. A few other species (identified as M. laciniatus, M. platycalyx, M. nudatus, and M. tilingii) were added by Sweigart et al. (2003) in order "To reconstruct the evolutionary history of the M. guttatus complex," "to understand the divergence history of these closely related, potentially hybridizing species." Essentially the same data and analyses were used by Sweigart and Willis (2008), with the addition of M. sookensis populations. In Figure 3 of Sweigart and Willis (p. 2497, neighbor-joining tree of mAP3 sequences), the clade of "M. guttatus Group P" (bootstrap = 100%) is M. grandis. My speculative interpretation here of the mCYCA tree (their Fig. 2, p. 2496) is slightly different from an earlier one (Nesom 2012). * the large upper cluster (bootstrap = 66% in 2003, 92% in 2008) apparently includes typical M. guttatus (e.g., plants from Alaska and Mexico) as well as M. microphyllus (the Iron Mountain population). Two populations identified as "Mimulus platycalyx" are included –– these perhaps also are an expression/variant of M. microphyllus. Whether these particular M. guttatus and M. microphyllus cluster because of hybridization/introgression or because of common ancestry is not known. * the group of smaller clusters in the middle of the tree (bootstrap = 55-100%) are subgroups of plants that I perhaps would identify either as M. guttatus or M. microphyllus in the strict sense, but they are primarily from the central California Sierra and represent groups potentially segregated as distinct, presently undescribed species (for example, see Nesom 2012, p. 38, third paragraph under under discussion of Morphological Variants of E. guttata). The largest and uppermost cluster of these (bootstrap = 84%) might be M. microphyllus, if the abundance of samples reflects the relative abundance of natural populations. * the bottom cluster includes typical M. nasutus, M. laciniatus (PRG1), and Mimulus guttatus "divergent sequences." The 5 samples of the latter, which underlie the hypothesis of asymmetric introgression (see below), are from central California (San Benito, Solano, Stanislaus, and Tuolumne cos.); these plants might be an expression of M. microphyllus in the broad sense but it is equally possible that they are some other species, since M. laciniatus also is included without distinction in the cluster. The "divergent sequences" were removed from the data in the Sweigart and Willis analysis. * Samples identified as M. platycalyx, M. nudatus, and M. tilingii are not resolved as either more or less closely similar to the various clusters of "M. guttatus." Mimulus laciniatus clusters with M. nasutus in the mCYCA tree but is distinct from it in the mAP3 tree. Thus, while Mimulus guttatus in the broad sense clearly is variable among populations, a portion of the variability is found in (a) different species that should not be misleadingly confused with more strictly defined M. guttatus and (b) in entities potentially segregated in the future as distinct species. An understanding of species-wide genetic diversity in Mimulus nasutus has a similar problem in interpretation. In Modliszewski & Willis, Fig. 4, two populations are mapped as M. nasutus but instead are probably other species. Both have the "red" haplotype otherwise characteristic of M. guttutus and never found in M. nasutus. The Colorado population is far out-of-range of M. nasutus and probably is M. hallii Greene; correspondingly, the California population is likely to be M. Nesom: Status of Erythranthe sookensis 12 arvensis Greene, closely related to M. hallii (see maps and comments in Nesom 2012, 2013). Both M. hallii and M. arvensis are annuals with autogamous flowers like M. nasutus, which may be the reason they were identified as the latter. Hybridization and introgression between Mimulus nasutus and M. guttatus Natural hybridization between Mimulus nasutus and "Mimulus guttatus" has been documented and discussed in various studies (e.g., Kiang 1973, using M. guttatus in the strict sense, from its description and an accompanying photo; Kiang & Hamrick 1978, using M. guttatus sensu stricto; Ritland 1991, apparently using M. guttatus sensu stricto; Fishman et al. 2002, using M. microphyllus from Iron Mountain, Linn Co., Oregon; Sweigart et al. 2008, also using M. microphyllus from Iron Mountain). Earlier, Munz (1959) had noted that M. nasutus and M. guttatus form hybrids. And as noted above, Benedict (1993) reported M. nasutus-microphyllus hybrids on Vancouver Island growing in close sympatry with the two parents as well as with the tetraploid M. sookensis. Martin and Willis (2007) studied hybridization between three populations each of Mimulus guttatus (all annual, John Willis pers. comm., and presumably correctly identified as M. microphyllus) and M. nasutus –– all from Stanislaus Co., California. They found that no natural hybrids occurred with M. microphyllus as the seed parent (0 of 3116 seeds examined, from numerous plants across all populations); experimental F1s from M. microphyllus as seed parent had greatly reduced viability and fertility. In contrast, about 1% of all naturally produced seeds of M. nasutus (33 of 3097 seeds examined) were highly fit F1 hybrids. Presumably all entities involved were diploid. Reproductive isolation between the two species is strong, but the opportunity and direction of limited introgression is from Mimulus nasutus into M. microphyllus. Backcrosses in nature of the Stanislaus County F1s occur with M. microphyllus but not M. nasutus. The hybrids have a greater overlap in flowering phenology with M. microphyllus and, because they produce relatively large flowers, flowers of the hybrids and M. microphyllus are visited by bees at a greater frequency than the much smaller ones of M. nasutus. At least in the Stanislaus County populations studied by Martin and Willis, stabilization of hybrids between Mimulus nasutus and M. microphyllus with the morphology and genetic constitution of M. sookensis was not observed and would be unexpected –– observed hybrids were morphological intermediates. Coincidentally, Stanislaus County is one of the counties from which populations match the mCYCA sequence of the non-nasutus parent of M. sookensis from the Vancouver region. Mimulus microphyllus and M. nasutus are broadly sympatric (Figs. 3 and 4) and natural hybrids between them appear to be relatively common. If indeed M. microphyllus is involved in the parentage of M. sookensis, and if F1s resemble M. sookensis, it seems reasonable to expect that M. sookensis-like tetraploids should occur more widely over the region of sympatry (for example, throughout most of California). But they apparently do not, and thus in agreement with Benedict's original reluctant and implicit assessment, while molecular evidence indicates that M. sookensis is such a hybrid, a reasonable account of the events associated with its evolutionary origin is lacking. Asymmetric introgression between Mimulus nasutus and M. guttatus/microphyllus Sweigart and Willis (2003) hypothesized that gene flow has occurred asymmetrically from autogamous Mimulus nasutus into allogamous M. guttatus. Among their samples identified as M. guttatus (apparently none suspected of being hybrid in morphology), "The finding of several M. guttatus sequences that share complete identity with sequences from M. nasutus suggests that recent asymmetric introgression [emphasis added] may have occurred. We argue that exceptionally high nucleotide diversity in M. guttatus is consistent with a long-term history of directional Nesom: Status of Erythranthe sookensis 13 introgression [emphasis added] from M. nasutus to M. guttatus throughout the divergence of these two species" (p. 2490). "Two lines of evidence argue ... that these divergent M. guttatus sequences are products of recent introgression from M. nasutus to M. guttatus. First, these M. guttatus sequences share near or complete nucleotide sequence identity with M. nasutus. Second, all four M. guttatus populations with M. nasutus-like sequences occur within a few kilometers of M. nasutus populations, so that some level of introgression is probable. One population exists in sympatry with M. nasutus (GMD), and the other three populations are in regions densely populated with M. nasutus" (Sweigart & Willis, p. 2490). Apart from the sympatry and associated "probable introgression," the asymmetric introgression hypothesis rests on the observation that the divergent sequence plants cluster with Mimulus nasutus in the neighbor-joining tree of mCYCA sequences. The distinct species M. laciniatus also clusters with M. nasutus in the same analysis, presumably reflecting their close common ancestry. The five samples with divergent sequences are from a close cluster of four central California counties: San Benito (population SBC), Solano (GMD), Stanislaus (DPC), and Tuolumne (GCC). These plants are all anuual (fide Seed Collections 2009 and other previously published studies in which these populations were studied). The other samples from each of these four populations (SBC, 7 of 8 samples; GMD, 6 of 7; DPC, 4 of 6; and GCC, 4 of 5) cluster within various other subgroups of M. guttatus in the broad sense. This pattern presumably could be interpreted as congruent with a hypothesis of introgression. It seems to be agreed that Mimulus nasutus and "M. guttatus" hybridize at a low frequency and gene flow into M. guttatus might indeed be occurring, although there is no evidence to indicate whether the documented putative introgression might be "long-term" or "recent" or even still on-going. The observations by Sweigart and Willis can be organized by another plausible explanation, also reasonably argued –– the plants sampled with "divergent M. guttatus sequences" perhaps are some other relatively narrowly distributed species and their nucleotide identity with M. nasutus was acquired from an evolutionary ancestor shared by both species. Mimulus pardalis should be investigated as it produces autogamous flowers and has an overall distribution at least overlapping with that of the divergent sequence populations. This scenario also might explain why a similar pattern of gene flow was not observed outside of this four-county area, since the sampling of M. guttatus in the broad sense covered a much broader region. Whether asymmetric introgression or common ancestry (or some other process) better accounts for the observed patterns presumably might be resolved by accurate identifications of the sampled plants. Vouchers were not prepared as part of the study but seed samples apparently exist for plants and populations analyzed (Seed Collections 2009) and plants presumably might be grown to maturity and identified. Does evolution repeat itself in polyploid populations of independent origin? The question (from Soltis et al. 2009) posed in the header might be viewed as apt with regard to Mimulus sookensis, especially if (as apparently implicitly or explicitly assumed in several publications, or as directly stated as in Modliszewski and Willis, p. 5295) two or more populations originated independently from M. nasutus x M. microphyllus crosses then underwent convergent morphological modification, all arriving at the appearance of M. sookensis. In the Tragopogon situation studied in detail by Soltis et al., allotetraploids T. mirus (T. dubius × T. porrifolius) and T. miscellus (T. dubius × T. pratensis) have formed repeatedly following introduction of the three diploid parents to the USA. Biology of the Tragopogon entities, however, differs from that in the Nesom: Status of Erythranthe sookensis 14 Mimulus situation in significant aspects –– parents of the hybrids are clearly identified and each of the two allotetraploids remains morphologically intermediate between its parents. In Tragopogon, genomes of the independently formed hybrid populations converge (evolution repeating itself) –– "Both allotetraploids exhibit homeolog loss, with the same genes consistently showing loss, and homeologs of T. dubius preferentially lost in both allotetraploids. We have also documented repeated patterns of tissue-specific silencing in multiple populations of T. miscellus. Hence, some aspects of genome evolution may be “hardwired,” although the general pattern of loss is stochastic within any given population" (Soltis et al. 2009, Abstract). Whether Tragopogon-like convergent genetic processes may occur among populations or population systems of M. sookensis remains to be seen. In any case, the query posed by Benedict et al. –– "how these interspecific polyploid hybrids between M. guttatus and M. nasutus all came to have the appearance of M. nasutus" –– may rest on unjustified supposition, since both parents are not known nor is it established that the contemporary tetraploids are morphologically modified from the original hybrids. Modliszewski and Willis (p. 5295) found evidence of duplicate gene copy loss in Mimulus sookensis, observed interfertility among independently derived populations that suggested to them that multiple origins were followed by hybridization among populations, and they believed that populations converged toward a common phenotype nearly identical to that of M. nasutus. The scenario they found most likely to account for the convergence (e.g., p. 5294) apparently includes these general phases (as I interpret their comments): (1) original formation of tetraploid hybrids intermediate in morphology between M. nasutus and M. microphyllus; (2) gene-silencing ("gene copy loss") of microphyllus-like genes with subsequent expression only of nasutus-like genes; and (3) production of a nasutus-like phenotype among all populations by gene flow across the entire geographic range of the tetraploids. Most parsimoniously, the whole set of requisite gene-silencing events occurred only once (vs. two or more independent occurrences) and gene flow occurred at least between the now apparently disjunct northern and southern population systems. Regardless of how many times the set of gene-silencing events occurred, "many genes of small to moderate effect on floral characters" (see above) and many vegetative genes seemingly were modified either in nearly perfect concert or else as a remarkably broad pleiotropic phenomenon probably not matched in any other known example. The convergence toward M. nasutus in such a scenario also would assume that the gene-silencing overwhelmed the tendency suggested by Sweigart and Willis (2003) and documented by Martin and Willis (2007) for introgression to occur between M. nasutus and M. microphyllus in the direction of M. microphyllus. Potential significance of the fixed heterozygosity characteristic of M. sookensis in relation to the effect of gene copy loss was not noted by Modliszewski and Willis. Taxonomic options If further evidence corroborates different parentage of at least each of two geographic population segments, then the name Mimulus sookensis presumably might apply only to the northern group of populations (whence the type) and two cryptic species would then be recognized. Such nomenclature would be more consistent with the evolutionary pattern. Or if more than two independent origins are unambiguously demonstrated, then the "sookensis" entity might be equally well characterized as a recurrent hybrid, especially if the origins were shown to be relatively recent (although there seems to be no evidence for that). On the other hand, multiple cytotypes are recognized in many species and this is a reasonable taxonomic approach (as adopted here) for M. nasutus and M. sookensis, which so overlap in morphology that a consistent separation is impossible. Informal use of the name M. sookensis in genetics research, however, is reasonable. Formal taxonomic recognition of Mimulus sookensis sets an interesting precedent in monkeyflower systematics, as numerous tetraploids have been discovered in M. guttatus, especially in Nesom: Status of Erythranthe sookensis 15 the southeastern part of its range (Arizona, Utah, Colorado, and New Mexico, mostly the Colorado Plateau; see Map 8 in Nesom 2012) and in Canada and Alaska. Because fewer Erythranthe species occur in these regions than along the Pacific coast of California to Washington, presumably it may be simpler to identify the parents of these tetraploids. Even in this reduced possibility, however, whether the M. guttatus tetraploids may be autoploids or segmental alloploids, or even interspecific hybrids (or a mix), is not known, notwithstanding repeated reference by Benedict et al. (p. 40) to all of them as autotetraploids. Following the precedent of M. sookensis, genetic interpretations might affect the taxonomy. Two populations of Mimulus nasutus are reported to have a chromosome number of n = 13 (rather than n = 14, which is otherwise characteristic of the species) (Mukherjee & Vickery 1960). Neither was mentioned by Benedict et al. (2012) among the populations of M. nasutus with chromosome counts. The California plants were studied by Vickery (1964, pp. 66–67) and found to be strongly isolated from conspecific populations and other species in experimental hybridizations –– Vickery noted that this apparent dysploid population probably "is on its own evolutionary pathway and is a separate species. Its accurate taxonomic designation must await a critical study of the pertinent literature." Formal recognition of a Mimulus species based solely on the criteron of reproductive isolation presently would be paralleled only in the Scottish M. peregrinus (see below); the situation in M. sookensis apparently is next closest. Further, though the two dysploid populations of M. nasutus probably originated independently, presumably if one were recognized at specific rank, the other might be included in the same species, following the suggested precedent of M. sookensis. California. Tuolumne Co.: 11 mi W of Yosemite Junction, ephemeral creek bed, Wild Cat Creek, 125 m, [no date], Vickery 168, culture 5327 (UT!). n = 13, 2n = 26. New Mexico. Dona Ana Co.: San Augustine Pass, by a small spring on the E side of the Organ Mts, ca. 5 mi from San Augustine Pass on a slope overlooking White Sands Rocket Testing Base, ca. 4500 ft, 30 Oct 1946, O. Norwell s.n., Vickery cult. 5018 (UT!). n = 13, 2n = 26. Another fertile allopolyploid with possible independent origins Another allopolyploid monkeyflower has recently been described (Vallejo-Marín 2012). Mimulus peregrinus Vallejo-Marín (hexaploid, 2n = 92) is known from a single locality along stream banks in the Lowther Hills of Scotland and is intermediate in floral and vegetative characteristics between typical M. guttatus (2n = 28, widely naturalized in Europe) and the South American native M. luteus L. var. rivularis Lindl. (2n = 60-62, also naturalized in Europe but less commonly so). The hexaploid is essentially identical in morphology to M. × robertsii Silverside (mostly M. guttatus × M. luteus var. rivularis), a triploid, highly sterile but vegetatively vigorous hybrid, perhaps mostly of horticultural origin (Vallejo-Marín & Lye 2013), that has become naturalized in scattered but extensive clonal populations in the British Isles. The hexaploid plants differ in chromosome number, high pollen and seed fertility, and increased pollen and stomata size. They were discovered "in a large population of M. × robertsii" (or "amongst a large population of M. × robertsii," or "alongside M. × robertsii"), where it seems likely that they arose through genome doubling of the triploid. Because of the widespread distribution of Mimulus × robertsii, Vallejo-Marín reckoned that hexaploids similar to M. peregrinus might occur in other localities, and the same possibility was earlier alluded to by Silverside (1988): "Parker (1975) reported a Mimulus population from the Lleyn peninsula in N. Wales with pollen fertility exceeding 70% and which on cytological grounds he regarded as a variant of [M. x robertsii]." Vallejo-Marín and Lye (2013), however, did not report encountering other hexaploids in their survey of 40 populations of naturalized Mimulus across Great Britain, including 300 individuals from within 17 populations of the larger set. Nesom: Status of Erythranthe sookensis 16 The triploid may have numerous independent origins. The type locality of Mimulus × robertsii "is a site where M. luteus var. rivularis occurs, along with at least three different clones of M. x robertsii. The occurrence of multiple clones of M. x robertsii is a phenomenon that is also linked with the presence of M. luteus var. rivularis in other localities, and it appears highly probable that they represent spontaneous local hybridisation" (Silverside 1988). Independent origins of the triploid also were implied by Roberts (1964), pointing out variability among various clones. Further complicating analysis of evolutionary origins is the observation by Silverside that triploids indistinguishable in morphology from M. x robertsii sometimes sometimes arise from crosses between M. guttatus and the South American M. cupreus Dombrain and have become naturalized. Further, as noted by Vallejo-Marín, plants identifiable as Mimulus × robertsii are "produced by crosses of M. guttatus with M. luteus var. rivularis, M. luteus var. variegatus or M. × smithii Paxton (the latter a hybrid between M. luteus var. rivularis and M. luteus var. variegatus, which is phenotypically very similar to M. luteus var. youngana) (Stace 2010)." Vallejo-Marín and Lye have provided additional comments regarding the origin of M. x robertsii and its inherent variability. Mimulus peregrinus and its putative genetic predecessor are brought into formal Erythranthe nomenclature with the combinations below. Still other hybrids remain named only in Mimulus. ERYTHRANTHE × ROBERTSII (Silverside) Nesom, comb. nov. Mimulus × robertsii Silverside, Watsonia 18: 211. 1988. TYPE: GREAT BRITAIN. Hethpool, College Bum, Northumberland, v.c. 68, GR 36/895.280, in hill stream, 10 Aug 1989, Silverside 1989/159 (holotype: E). Mimulus × robertsii was specified by Silverside as a hybrid between M. guttatus and M. luteus var. rivularis Lindl. [Erythranthe guttata and E. lutea (L.) Nesom var. rivularis (Lindl.) Nesom]. He noted that "The view is taken here that the 'luteus' parent is M. luteus var. rivularis Lindley, though there are good reasons for considering this taxon as not being conspecific with the typical M. luteus, which does not occur in Britain." ERYTHRANTHE PEREGRINA (Vallejo-Marín) Nesom, comb. nov. Mimulus peregrinus VallejoMarín, Phytokeys 14: 4. 2012. TYPE. UNITED KINGDOM. Scotland. Grown from seed collected in South Lanarkshire near Leadhills, on the banks of Shortcleuch Water, Vice county 77, alt. 360 m, 27 Aug 2011, M. Vallejo-Marín 11-LED-seed; vouchered as M. Vallejo-Marín 11-LED-seed-2-14 (holotype: E; isotypes: BM, K). Regarding each of these names, IPNI (mirrored by Tropicos) gives the following comment: "Contrary to Art. H5.2 ICBN, note 1. This taxon was designated at a rank inappropriate to its hybrid formula. This name would be approriate for the hybrid between Mimulus guttatus × M. luteus." The referenced Article from the ICBN (McNeil et al. 2006) is this: H.5.2 If the postulated or known parent taxa are of unequal rank the appropriate rank of the nothotaxon is the lowest of these ranks. Note 1. When a nothotaxon is designated by a name in a rank inappropriate to its hybrid formula, the name is incorrect in relation to that hybrid formula but may nevertheless be correct, or may become correct later. Because of the ambiguous genetic constitution and uncertainty about corresponding names for the non M. guttatus parent of the two named taxa proposed to be of hybrid ancestry, Vallejo-Marin and Lye (2013) have referred to it as "M. luteus sensu lato." Presumably, this meets the circumstances noted in the ICBN and both names can be taken as correct. As the sterile hybrid is identified as Mimulus × robertsii, it may be appropriate to identify the fertile one as "M. × peregrinus" or the latter might even be identified as "hexaploid M. × robertsii." Whether or not the hexaploids are considered as a species in an evolutionary context depends on one's species concept. They are isolated from the triploids but perhaps not from the putative parents and they are distinctive only in size differences necessarily measured with a compound microscope. In Nesom: Status of Erythranthe sookensis 17 the same vein, it seems unusual that Vallejo-Marín has suggested that the hexaploid plants be regarded as "Critically Endangered" –– others might view them as a non-native entity with increased capacity for invasiveness and recommend that they be eradicated. ACKNOWLEDGEMENTS This study has been supported by the Flora of North America Association in connection with preparation of the taxonomic treatment of Erythranthe. I am grateful to Fred Ganders, John Willis, and Jennifer Modliszewski for comments, to WTU and UBC for loans of specimens, and to the staff of BRIT for arranging the loans. LITERATURE CITED Barker, W.R., G.L. Nesom, P.M. Beardsley, and N.S. Fraga. 2012. A taxonomic conspectus of Phrymaceae: A narrowed circumscription for Mimulus, new and resurrected genera, and new names and combinations. Phytoneuron 2012-39: 1–60. Benedict, B.G. 1993. Biosystematics of the annual species of the Mimulus guttatus species complex in British Columbia, Canada. M.S. thesis, Univ. of British Columbia, Vancouver, Canada. Benedict, B.G., J.L. Modliszewski, A.L. Sweigart, N.H. Martin, F.R. Ganders, and J.H. Willis. 2012. Mimulus sookensis (Phrymaceae), a new allotetraploid species derived from Mimulus guttatus and Mimulus nasutus. Madroño 59: 29–43. Fishman, L., J.A. Kelly, and J.H. Willis. 2002. Minor quantitative trait loci underlie floral traits associated with mating system divergence in Mimulus. Evolution 56: 2138–2155. Griffiths, A.J.F. and F.R. Ganders. 1983. Wildflower Genetics, A Field Guide for British Columbia and the Pacific Northwest. Flight Press, Vancouver. Kiang, Y.T. 1973. Floral structure, hybridization and evolutionary relationship of two species of Mimulus. Rhodora 75: 225–238. Kiang, Y.T. and J.L. Hamrick. 1978. Reproductive isolation in the Mimulus guttatus-M. nasutus complex. Amer. Midl. Naturalist 100: 269–276. Lowry, D.B., R.C. Rockwood, and J.H. Willis. 2008. Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evolution. 62: 2196–2214. Martin, N.H. and J.H. Willis. 2007. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution 61: 68–82. McNeil, J. et al. (prepared and edited by). 2006. International Code of Botanical Nomenclature (Vienna Code). Regnum Vegetabile 146. A.R.G. Gantner Verlag KG. Koeltz Scientific Books. Modliszewski, J.L. and J.H. Willis. 2012. Allotetraploid Mimulus sookensis are highly interfertile despite independent origins. Molec. Ecol. 21: 5280–5298. Mukherjee, B.B. and R.K. Vickery, Jr. 1960. Chromosome counts in the section Simiolus of the genus Mimulus (Scrophulariaceae). IV. Madrono 15: 239–245. Munz, P.A. (in collaboration with D.D. Keck). 1959. A California Flora. Univ. of California Press, Berkeley. Nesom, G.L. 2012. Taxonomy of Erythranthe sect. Simiola (Phrymaceae) in the USA and Mexico. Phytoneuron 2012-40: 1–123. Nesom, G.L. 2013a. Observations on habit and duration in populations of Erythranthe microphylla and E. guttata (Phrymaceae). Phytoneuron 2013-68: 1–8. Nesom, G. L. 2013b. New distribution records for Erythranthe (Phrymaceae). Phytoneuron 201367: 1–15. Parker, P.F. 1975. Mimulus in Great Britain – a cytotaxonomic note. New Phytol. 74: 155–160. Ritland, K. 1991. A genetic approach to measuring pollen discounting in natural plant populations. Amer. Naturalist 138: 1049–1057. Roberts, R.H. 1964. Mimulus hybrids in Britain. Watsonia 6: 70–75. Nesom: Status of Erythranthe sookensis 18 Seed Collections. 2009. Seed collections –– seeds from wild populations and permanent mapping populations (last seed database update noted as 22 Mar 2009). Mimulus Community 2013. Announcements, The genus Mimulus, Mimulus genome, people, seed collections, techniques. OpenWetWare. <http://openwetware.org/wiki/Mimulus_Community> Silverside, A.J. 1990. A new hybrid binomial in Mimulus L. Watsonia 18: 210–212. Soltis, D.E., R.J. Buggs, W.B. Barbazuk, P.S. Schnable, and P.S. Soltis. 2009. On the origins of species: Does evolution repeat itself in polyploid populations of independent origin? Cold Spring Harb. Symp. Quant. Biol. 74: 215–223. <http://symposium.cshlp.org/content/early/2009/08/16/sqb.2009.74.007.abstract> Stace, C. 2010. New Flora of the British Isles (ed. 3). Cambridge Univ. Press, Cambridge. Sweigart, A.L. and J.H. Willis. 2003. Patterns of nucleotide diversity are affected by mating system and asymmetric introgression in two species of Mimulus. Evolution 57: 2490–2506. Sweigart, A.L., N.H. Martin, and J.H. Willis. 2008. Patterns of nucleotide variation and reproductive isolation between a Mimulus allotetraploid and its progenitor species. Molec. Ecol. 17: 2089–2100. Vickery, R.K., Jr. 1964. Barriers to gene exchange between members of the Mimulus guttatus complex (Scrophulariaceae). Evolution 18: 52–69. Vallejo-Marín, M. 2012. Mimulus peregrinus (Phrymaceae): A new British allopolyploid species. Phytokeys 14: 1–14. Vallejo-Marin, M. and G.C. Lye. 2013. Hybridisation and genetic diversity in introduced Mimulus (Phrymaceae). Heredity 110: 111-122.