* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download EMR notes
Survey
Document related concepts
Transcript
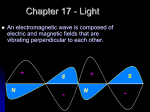
UNIT THREE – ELECTROMAGNETIC RADIATION LESSON 1-History: Describe, qualitatively, how all accelerating charges produce EMR Explain the propagation of EMR in terms of perpendicular electric and magnetic fields that are varying with time and travelling away from their source at the speed of light Faraday believed in unity in nature and that there was a relationship between light, electricity and magnetism. He however, lacked the mathematics to prove this. James Clerk Maxwell, in 1860’s, developed the mathematical theory known as the theory of electromagnetic radiation. There are 4 principles to the theory; Oersted and Faraday established the first two: 1. Oersted: An electric current will produce a magnetic field (motor effect) 2. Faraday: A magnetic field can produce an electric current (generator effect) Maxwell added to these principles so that they apply to electric and magnetic fields in conductors, insulators, and even in free space. Maxwell’s principles are: 3. A changing electric field in space produces a magnetic field of a magnitude that is proportional to the RATE of change of the electric field. BE/t 4. A changing magnetic field in space produces an electric field of magnitude proportional to the RATE of change of the magnetic field. EB/t To explain these last two principles, Maxwell used the concept of displacement current. (movement of electrons in a substance because of induction-this is a temporary current) To maintain a current the conditions around the substance must continually change. Maxwell assumed that these principles could exist in free space!! (outer space-no atmosphere) According to his theory, a changing magnetic field produces a changing electric field, which produces a changing magnetic field and so on. It’s an unending sequence of events. Since magnetic field around a current-carrying conductor depends on the current (rate of flow of charge), a constant current would have a constant magnetic field. If the current changes, so does the magnetic field. Therefore, according to Maxwell’s theory, an accelerating charged particle will produce a changing magnetic field, which will produce the changing electric field, etc. Maxwell called this unending sequence of events the electromagnetic wave (EMR)!!! It’s vibrating electric and magnetic fields generate one another. The fields are perpendicular to each other at any point and perpendicular to the direction of propagation of the wave. http://en.wikipedia.org/wiki/Electromagnetic_radiation **Maxwell calculated the speed of EMR to be 3.00 x 108 m/s. Just a few years after, the speed of light was also measured to be 3.00 x 108m/s. Maxwell concluded that light is a form of electromagnetic radiation! (as Faraday had suspected) **Maxwell predicted that EMR waves of many different frequencies could exist. All such waves would travel through space at the speed of light and have the same properties of light; they would all reflect, refract, diffract, interfere and be polarized. HEINRICH HERTZ Maxwell never produced or detected these waves, he only predicted their existence. IN 1886, Hertz successfully did both of the above. Using a spark gap across which electric charges move rapidly back and forth (accelerating charged particles), Hertz generated EMR waves whose frequency was about 109 Hz. He was able to detect these waves some distance away using a loop of wire as a receiver (antenna). Hertz was able to show that theses waves traveled at a speed of 3.00 x 108m/s and have light characteristics. Hertz called these waves radio waves. Marconi who became the first to transmit radio waves across the Atlantic Ocean in 1901 used his discovery. LESSON 2 – EMR SPECTRUM Compare and contrast the constituents of the electromagnetic spectrum on the basis of frequency and wavelength There is a broad range of continuous frequencies for EMR called the electromagnetic spectrum. It may be described in terms of wavelength and energy, as well as frequency – all of which are mathematically related! http://www.youtube.com/watch?v=bjOGNVH3D4Y Visible light has a very small part of the EMR spectrum. Its range is between 7.0x10-7 (700 nm) and 4.0x10-7 (400nm). Recall the universal wave equation: v = Where v=speed of the wave (m/s) =wavelength (m) =frequency (Hz-revolutions per second) This equation applies to EMR as well. The speed of EMR is given the symbol c, which is 3.00x108 m/s. c= Thus, the greater the frequency of EMR the lower its wavelength. (inverse relationship) pg 189-193 **All EMR is produced by accelerating charged particles. Radio waves (including television signals and cell phones) and microwaves (radar) are produced by the oscillations of electric current. Microwaves are extremely high frequency radio waves of wavelengths of 12.2 cm and frequency 2.45x109 Hz. It causes water molecules to vibrate. This increases kinetic energy and therefore temperature. Radar signals are high frequency microwaves. Cell phones use microwaves as does satellite communication. Infrared radiation is produced by molecular vibrations. Infrared is associated with heat. The hotter an object, the greater the infrared radiation coming from the object. It is used in remote controls, heat lamps, burglar alarms, and infrared cameras. Visible light and UV are produced by the transitions of electrons in atoms. Visible is 400-700nm in wavelength and runs respectively from violet, indigo, blue, green, yellow, orange and red (ROYGBIV backwards). Ultraviolet means beyond violet and has a lower wavelength than 400nm. It causes skin cancer and sunburn and is blocked somewhat by the ozone layer in the atmosphere. X-rays are produced by high-energy electrons undergoing rapid deceleration by hitting a target. Used in medicine and airport security. Can cause cell damage. Gamma rays (or gamma radiation)are produced by the decaying nuclei of unstable atoms. Carries the most energy of all EMR (lowest wavelength – highest frequency) and therefore causes the most damage, so it is the most dangerous. Its high energy can cause cancer, but it is also used in medicine to destroy cancer cells (radio-therapy). *Some of these forms may overlap in frequency and wavelength, but are classified by their method of production (above) instead of their fundamental properties of frequency and wavelength. Activity: Determine the wavelength of EMR Purpose: To calculate the speed of EMR (microwaves). Background: Microwave ovens produce EMR waves by accelerating electrons in a device called a magnetron. The electrons are accelerated back and forth at a frequency of 2.45x109 Hz (2.45GHz). Also, reflection of the waves within the microwave oven occurs to produce standing waves. (draw) You can determine the wavelength of a microwave by placing a slice of cheese in a microwave oven and microwaving it for approximately 10s. (You may have to experiment with the time a little bit). If the microwave oven has a rotating plate, you should remove it. The cheese will begin to melt only at the antinodes of the standing wave. By measuring the distance between the antinodes, you can determine the wavelength. (Remember = 2 times the distance between antinodes) Data: Frequency of microwaves______________ Distance between antinodes______________ Microwave wavelength______________ Speed of EMR _________________ Percent error________________ LESSON THREE– SPEED OF LIGHT EXPERIMENTS Explain qualitatively, various methods of measuring the speed of EMR Calculate the speed of EMR, given data from a Michelson-type experiment Design an experiment to measure the speed of light Late 16th century: first recorded attempt to measure the speed of light. Galileo and an assistant measured the distance across a field and took lanterns to opposite sides. Galileo opened the lantern and when the assistant saw it they opened their lantern. When Galileo saw the light from the second lantern he stopped the timer. This method was not successful as the speed of light is too high and the reaction time error was too great. v=d/t 1675: first successful measurement of the speed of light. Roemer and Huygens. A time of astronomy. Roemer made predictions about astronomical events (Jupiter and its moon) and for the most part was correct. However, on some occasions (6 months later) it was off by a small amount. Huygens interpreted this as the time required for the light to travel across Earth’s orbit. The orbital diameter was approximated and the extra time that Roemer was off by was used in the equation v=d/t to find the speed of light. It was found to be 2.26 x 108 m/s. 19th century: it was determined that to get an accurate speed of light, long distances or short time periods were required. Fizeau and Foucault measure the speed of light using short time periods. A rotating slotted wheel was used and light was passed through it and had to return through it to be seen. Adjusting the rps or rpm of the wheel for maximum brightness, allowed for determining the speed of light. mirror d Foucault used this method to measure the speed of light in water. This measurement had great significance since particle theory of light stated that it should speed up in water and wave theory said light should slow down in water. Foucault found that the speed of light in water was slower than in air supporting the wave theory. 1880: A.A. Michelson measures the speed of light using a rotating mirror and a fixed mirror over a large distance. light source 30 km observer Light reflected from the eight sided mirror to a fixed mirror 30 km away and returned to the eight-sided mirror. When the eight-sided mirror was rotated, the fixed mirror would receive flashes of light. The flash would return to the eight-sided mirror and would be picked up by the viewing instrument. In order for the light to be picked up by the viewing instrument, the eight-sided mirror had to rotate exactly one side (1/8), two sides (2/8), etc, of a revolution while the light travelled from one mirror to the other and back. Knowing the speed of rotation of the eight-sided mirror, the speed of light could be calculated. c=299,792,458 m/s. At this speed, light travels 9.46 x 1012 km in a year. This distance is a light-year. Michelson received a Nobel Prize for this experiment. Pg 195-202 LESSON FOUR: PROPERTIES OF EMR Describe, quantitatively, the phenomena of reflection and refraction, including total internal reflection All forms of EMR have the same properties. Since we can view light without additional special equipment, we will study the properties according to light. The properties do vary, however, according to wavelength. An example of this is that the longer the wavelength, the greater the diffraction. (However, all EMR diffracts) The properties of EMR include: Reflection Refraction Diffraction Interference Polarization 1. Reflection: Both waves and particles will reflect according to the law of reflection: the angle of reflection equals the angle of incidence. r = i Plane Mirror-flat smooth mirror: The incident ray is the incoming ray. The reflected ray is the outgoing ray. The normal is a line perpendicular to the reflecting surface. The angles of incidence and reflection are both measured with respect to the normal. Light obeys this law, however, this does not help us in determining if light is a wave or particle since this law is a property of both forms of energy. Properties of the image from a plane mirror: (read pg 654-656) Virtual Image – an image formed by diverging light rays. It is always on the opposite side of the mirror from the object. Position- the image position is equal to the negative of the object position. The negative sign indicates that the image is virtual. Height- image height is equal to object height Orientation- the image has the same orientation as the object (top and bottom) but has a front-to-back reversal (mirror image-right and left are reversed). Some of pages 203-213 Assignment: 1. If the angle of incidence of a ray of light to a mirror is 50.0o, what is the angle of reflection from the mirror? 2. IF the angle of incidence of a ray of light to a mirror is 20.0o, what angle does the reflected light ray make with the mirror? 3. If an incident ray of light makes an angle of 58o with a mirror, what is the angle between the incident ray and the reflected ray? 4. A ray of light is reflected in series off of two mirrors, A and B, as shown in the diagram below. What is the angle of reflection from mirror B? LESSON 5 – REFLECTION FROM CURVED MIRRORS Describe, quantitatively, simple optical systems, consisting of only one component, for both lenses and curved mirrors Use ray diagrams to describe an image formed by thin lenses and curved mirrors There are two types of curved mirrors; concave and convex. Concave mirrors have a ‘caved in’ shape: convex mirrors have a ‘pot belly’ shape. Of course it depends which side is mirrored and which side you are standing on! Concave mirrors are used in reflector telescopes and satellite dishes to reflect television signals. The mirror in the back of the drugstore is a convex mirror. Rear view/side mirrors on some vehicles may also be convex. A convex mirror has a VIRTUAL focus and is called a DIVERGING MIRROR. A concave mirror has a REAL focus and is called a CONVERGING MIRROR. SPHERICAL ABERRATION – all spherical mirrors have a defect called the spherical aberration. Rays of light parallel to the principle axis that reflect NEAR THE EDGE OF THE MIRROR do not reflect through the principle focal point. To correct this problem, the shape of the mirror maybe adjusted to a parabolic shape, or their size is kept relatively small compared to the radius of curvature. We will assume that the mirrors are relatively small and the problem is not significant. RAY DIAGRAMS – CURVED MIRRORS: The position, size, and nature of an image produced by a curved mirror can be found by a scale ray diagram. Use the following steps: 1. Draw the mirror, including its principle axis, centre of curvature, and principle focal point. 2. Place a vertical arrow on the principle axis to illustrate the position and size of the object. 3. Draw two rays from the tip of the object arrow to the mirror. Choose from the following 3 types of rays: a. A ray from the head of the object and parallel to the principle axis that then reflects through (or appear to diverge from) the principle focal point. b. A ray passing through the centre of curvature and the head of the object, which will reflect, back along the same path. c. A ray passing through the head of the object and the focal point (or directed at the focal point) that then reflects parallel to the principle axis. Where the reflected rays meet (real image) or appear to diverge from (virtual image), is the position of the image. A vertical arrow can be drawn from the principle axis to the meeting point described to represent the position and size of the image. Convex: Concave: Characteristics of the images: Real or virtual Erect or inverted Larger, smaller, or the same size as the real object Pg 225-227 Summarize properties of images based on object positions: The numerical values produced by ray diagrams may not be as precise as those produced mathematically. They are used to describe characteristics of the image. MATHEMATICS OF MIRRORS: To indicate the difference between images that are real or virtual, focal points that are real or virtual, images that are erect or inverted, we need to incorporate some sign conventions into the mathematics. Real focal points: f (focal length) is positive Real images: di (distance of the image) is positive Erect images: hi (height of image) is positive Virtual focal points: f is negative Virtual image: di is negative Inverted image: hi is negative You might note that ALL real images are inverted, and all virtual images are erect!!!! Formulas: 1. Magnification: M = hi/ho or di/do where M = magnification (how many times bigger the image is than the object) hi= image height ho = object height di = image distance from the mirror do = object distance from the mirror therefore: hi/ho = -di/do * the negative sign is part of the formula and necessary! 2. Mirror equation: 1/f = 1/do +1/di Examples: Pg 228-229 LESSON SIX– REFRACTION AND SNELL’S LAW Describe, qualitatively and quantitatively, how refraction supports the wave model of EMR using the wave equation for refraction. http://www.youtube.com/watch?v=FAivtXJOsiI Refraction is the bending of a wave as it changes velocity. A change in velocity is caused by a change in the medium (material) through which the wave travels. Waves bend when they change medium/speed, particles don’t. Similar to reflection, the angles of incidence and angle of refraction are measured with respect to the normal. However, the relationship between the refracted angle and the incident angle is more difficult to see. It was worked out by Willebrord Snell and is therefore referred to as the law of refraction or SNELL’S LAW: sin1/sin2 =v1/v2 = 1/2 = n2/n1 Where ‘n’ is a constant that compares the speed of light in the medium to the speed of light in air or a vacuum. ( n=c/v ) This is called the index of refraction for the medium. The greater the index of refraction is, the greater the change in direction. Diamond has an index of 2.42 while ice has an index of 1.31. Note that the index ratio is an inverse ratio to the others!!! n for air/vacuum is 1.00 and the speed of light in the same is 3.00 x 108m/s. KNOWN QUANTITIES!! When light slows down (smaller speed), the angle will be smaller and the wavelength will be smaller. (higher index!!) Any time a wave hits a boundary between mediums, you get a combination of reflection and refraction. We are going to focus on the refraction: What happens to the frequency of waves or light when it passes from one medium to another? Does a mechanical wave speed up of slow down in a denser medium? (think water/sound waves). What does EMR do? What do particles do? Critical angle! When light passes from a medium that has a high index of refraction (water) into a substance that has a lower index of refraction (air), the light speeds up and bends away from the normal. An angle of incidence can occur such that the refracted angle is 90o and this angle that creates a 90o refraction is called the critical angle. Any incident light ray at an angle bigger than the critical angle will cause reflection only! This result is called total internal reflection. It only occurs when light from a medium with a high index hits a barrier to one with a lower index. Some of pages 203-213 EXAMPLES OF REFRACTION; A pool of water is deeper than it appears; this is because the light waves in the water are shorter than in the air. The actual depth of the water is 1.33 times great than the apparent depth! The highway ahead of you when you drive your car appears to be wet, but you never reach the wet road; this is a mirage. The image that you see is a reflection of the sky on the hot layer of air above the road. The index of refraction of hot air is less than the index of refraction of cool air; therefore, the light passes from cool air (high index) into hot air (low index). Total reflection can occur – you will see a reflection of the sky. The twinkling of stars or distant lights is due to refraction. The direction the light travels changes as it passes through air of different temperatures. Because this air is in motion, it will change the position that the star or light appears to be – but only slightly. You see the sun and the sunlight for a few minutes before it rises and after it sets. Earth’s atmosphere refracts the light from the sun. Fibre optics uses total internal reflection. As the light travels down these glass fibres, it cannot escape. Light enter at one end of the fibre and each time it strikes the surface the angle of incidence is larger than the critical angle. The light is therefore kept within the fibre. Fibre optics is replacing electric circuits in communication technology, such as cable television and telephone. Physicians can even use fibre optics to look inside your body. LESSON SEVEN– LENSES Lenses work on the principle of refraction much like mirrors are reflection. Similar to mirrors there are two types of lenses: concave and convex. Any lens that is thinner at the center than at the edges is a concave lens. These lenses are ‘caved in’ on one or both sides. They are called a diverging lens (concave is diverging!!! Backwards to mirrors!) because when light passes through it, the light spreads out. Any lens that is thicker at the centre than at the edges is a convex lens. They have a pot-belly shape on one or both sides. A convex lens is a converging lens because when light passes through it, the light comes together. (Again! backwards to mirrors) Eg. Our examples will focus on double concave and double convex lenses for simplicity, and they will be referred to as concave or convex lenses. Because light can travel through a lens in either direction, a focal point is indicated on both sides (principle and secondary) of the lens at the same distance from the lens. In a convex lens the principle focal point is a real focal point (on the refracted side), but in the concave lens the principle focal point is a virtual focal point (on the incident ray side). In mirrors the focal point was ½ the radius of curvature, but in lenses the material of the lens as well as the curvature affect the distance of the focal length from the optical centre. The lower the index of refraction of the material the longer the focal length. The greater the radius of curvature, the longer the focal length. Ray Diagrams: The position, size and nature (real or virtual) of images can be found by a scale diagram much like mirrors. Use the following steps; 1. Draw the lens, including the principle axis and both focal points. 2. Place a vertical arrow on the principle axis to illustrate the position and size of the object. 3. Draw two rays from the tip of the object arrow to the lens by choosing from the following: a. A ray parallel to the principle axis, refracting through (or will appear to diverge from) the principle focal point. b. A ray passing through the optical centre, which does not change direction. c. A ray passing through the secondary focal point (or directed toward it), that refracts parallel to the principle axis The point where the REFRACTED rays meet (real image) or appear to diverge from (virtual image) is the position of the image. If a vertical arrow is drawn from the principle axis to the point described, the position and size of the image will be indicated. The characteristics of the image are described the same way as with mirror images. I.e. Erect or inverted, real or virtual, larger smaller or same size. Convex: Concave: Pg 216-217 MATHEMATICS OF LENSES: -More accurate way to find image characteristics Sign conventions are the same as with mirrors: Real focal points: f (focal length) is positive Real images: di (distance of the image) is positive Erect images: hi (height of image) is positive Virtual focal points: f is negative Virtual image: di is negative Inverted image: hi is negative You might note that ALL real images are inverted, and all virtual images are erect!!!! Equations are the same! Eg. 1 A glowing object 2.5 cm tall is placed 15 cm from a convex lens. If the lens has a focal length of 7.5 cm, what a) is the distance of the image from the lens? b) Is the size of the image? c) Are the characteristics of the image? Eg. 2 A glowing 4.0 cm tall object is placed 9.0 cm from a concave lens. If the lens has a focal length of 5.0 cm, what a) is the distance of the image from the lens? b) Is the size of the image? c) Are the characteristics of the image? Pg 218-222 Applications of Lenses and Mirrors LESSON EIGHT– Wave Nature of Light (Diffraction/Interference and Poisson’s Spot) Describe, qualitatively, diffraction, interference and polarization Describe, qualitatively, how the results of Young’s double-slit experiment support the wave model of light Solve double-slit and diffraction grating problems using the formulas Demonstrate the relationship among the wavelength, slit separation, and screen distance using empirical data and algorithms In the 17th century there was debate about the nature of light. Light is a form of energy, and energy can be transmitted by means of particles or by means of waves. Sir Isaac Newton believed that light traveled by particles while Christian Huygens believed that it traveled as waves. During the 1st part of the 19th century, a number of developments supported the wave theory. These were: Young’s double slit experiment The discovery of Poisson’s spot The measurement of the speed of light in water Polarization DIFFRACTION: Diffraction and wave interference are fundamental properties of waves. Neither diffraction nor wave interference can be explained using the particle model since particles do not exhibit these behaviors. Therefore, it can be concluded that if light exhibits these properties, it must travel as a wave. Diffraction- energy bends (or spreads out) as it passes through a small opening, or when it moves past an obstacle. From studies of water waves, it is known that the extent of diffraction depends on the wavelength of the wave and width of the opening. The longer the wavelength is, the greater the diffraction; the smaller the opening, the greater the diffraction. Light has a very small wavelength in the order of 10-7m. Therefore, you have to use a very small opening, or slit, in order to observe significant diffraction. INTERFERENCE: Wave interference is another fundamental property of waves. Particles do not interfere constructively or destructively. When a crest from one source meets a crest from another source, their energies combine to displace the medium. Constructive interference – the amplitude of the combined waves is larger. Destructive interference – the amplitude of the combined waves is smaller. If you were to look in the glass side at the end of the ripple tank, you would see alternating constructive and destructive interference points. This is a diffraction pattern! The maxima and minima occur in alternating patterns. Thomas Young applied the concept of diffraction pattern to light. In 1803, he demonstrated it with the following procedure 1. He blackened a glass slide and put two very narrow scratches on the slide. 2. He passed monochromatic light through these scratches. 3. From this setup, Young observed a diffraction pattern on a screen. This shows that light both diffracts and interferes and is evidence of light as a wave. Yet, at the time light as a wave was not accepted by all scientists. λ = dsinθ/n maxima/bright fringe or band/antinode If d is small relative to l, then the angles are small and the two angles are close to equal. At this point we can assume several things: that the two angles are equal, and that sin θ = tan θ. Because of this, we can use the equation λ = dx/nl but recognize that this is only accurate if the angle is less than 100. **maximum interference(bright spots are brightest) occurs when the size/spacing of the opening matches the wavelength of EMR. Diffraction gratings: Pg237-243 POISSON’S SPOT: (see shared physics lab video) In 1818 Fresnel wrote a paper about light as a wave and that a shadow of a sphere illuminated by monochromatic light should have a bright spot at the centre (due to diffraction). Poisson argued that light did not travel as a wave and thought this idea was ridiculous. Arago tested Poisson’s prediction and it was demonstrated that there is indeed the existence of this bright spot. The centre of a spherical or circular object is equidistant from all points along the circumference and therefore the light waves should reach the centre of the shadow from all around the circumference in phase, resulting in constructive interference (a maxima). This only occurs, however, if the circular (spherical) object is small enough that light can diffract to the centre. It works best if the circular/spheres diameter matches the wavelength of EMR – maximum diffraction and brightest spot. LESSON NINE– POLARIZATION Not only is there evidence that light travels as a wave but there are two types of waves – transverse and longitudinal. Transverse waves can be polarized. Light can be polarized. Therefore, light is most likely a transverse wave. Transverse waves can be polarized because their particles vibrate perpendicular to the direction of the energy flow. They therefore can vibrate in any plane and when vibrating in all planes are called non-polarized light. When light goes through a polarizing filter, it is restricted to one plane and therefore polarized. If two filters are used, they can be rotated to adjust the amount of light passing through. When the filter axes are perpendicular to each other, they block all of the light. This is evidence that light is a not only a wave, but a transverse wave. Much of the glare reflected from horizontal surfaces is polarized horizontally. This is why polarized sunglasses are used to reduce glare. The polarization axis is vertical – therefore greatly reducing the glare. Three-dimensional movies also use polarization to give the appearance of depth. Two pictures are taken from slightly different angles. These pictures are then projected by using two projectors. One of these is fitted with a vertical polarizer and the other with a horizontal polarizer. When you watch the pictures wearing polarized glasses, one of the lenses is vertical and the other is horizontal. This allows your two eyes to observe the original scene from slightly different angles. This explanation for polarization was given by Young and Fresnel in 1820. Up until that time, the wave model of light supported the concept that light travelled by longitudinal waves. Pg232-234 LESSON TEN – DISPERSION OF LIGHT Compare and contrast the visible spectra produced by diffraction gratings and triangular prisms. White light is composed of 7 colours of light; red, orange, yellow, green, blue, indigo, and violet. The dispersion of light is the separation of white light into those components. It can be separated by means of refraction or diffraction. Dispersion by means of refraction: The order of the colours produced by the spectrum should show that violet is refracted the most and red is refracted the least! (lower λ (violet) refracts the most – it has a lower speed in glass than higher wavelengths (red), smaller angle of refraction, larger change in direction) Violet and blue light also scatters more by molecules in the atmosphere producing the image of a blue sky. Dispersion by means of diffraction: The order of the spectrum produced should show violet at the inside of the 1st maxima and red at the outside of the first maxima! (higher λ (red) diffracts the most; λ = dsinθ/n) This is why the initial light seen from a sunrise is red/orange in color and the last light from sunset is also red/orange as it diffracts the most around the Earth. Pg232-234 Wave properties review pg 246 LESSON ELEVEN – WAVE PARTICLE DUALITY Read PG 702-705 (Pearson) Define the photon as a quantum of EMR and calculate its energy Classify the regions of the electromagnetic spectrum by photon energy Classical physics could not explain blackbody (incandescent – perfect radiator of light as well as a perfect absorber of all frequencies) radiation using wave model of light. When hot solids and gases are heated they emit EMR. At low temperature they emit IR and at high temperatures they emit most of the visible spectrum as well as UV. The brightness is mainly dependent on the temperature so that as the temperature rises the spectrum shifts to higher frequencies. Maxwell’s theory of EMR predicts that radiation is causes by the oscillation of electric charges (changing fields) and as the temperature increases, so too should the intensity and frequency at a steady rate –classical physics. However, this was not observed. In 1900, Max Planck derived an equation to describe the blackbody radiation but in order to do that he had to hypothesize that an oscillator, including vibrating atoms and molecules is quantized. That is, the oscillator can only have certain values of energy. The energies allowed are given by E=nhf Where E = energy n = positive integer h = Planck’s constant (6.63 x 10-34 Js) f = frequency of vibration Planck’s hypothesis was the beginning of the quantum theory. It seemed too radical at the time and was not generally accepted. It was compared to saying that a swinging pendulum could only have certain energies and could only increase in height by certain intervals. In 1905, Albert Einstein used Planck’s hypothesis to include light energy. He explained that energy in light was not continuous but quantized. The energy of a quantum depended on the frequency of the light (or EMR). A ‘quantum’ is now called a ‘photon’. E = hf or hc/λ The quanta in the red region have lower energy than quanta in the UV region. This energy is often expressed in eV for photon energy and the conversion is on your data sheet. Planck’s theory has two important implications” 1. EMR waves do not transmit energy continuously but rather in small packages called quanta – later known as photons 2. Physical objects do not vibrate with random energy because the energy is restricted to certain discrete values. This is seen only with microphysics – atomic physics. It is insignificant to macrophysics (large body objects) Pg252-256 LESSON TWELVE – Einstein and the PHOTOELECTRIC EFFECT Describe the photoelectric effect in terms of the intensity and wavelength or frequency of the incident light and surface material Predict the effect, on photoelectric emissions, of changing the intensity and/or frequency of the incident radiation or material of the photocathode Describe, quantitatively, photoelectric emission, using concepts related to the conservation of energy Describe the photoelectric effect as a phenomenon that supports the notion of the wave-particle duality of EMR Analyze and interpret empirical data from an experiment on the photoelectric effect using a graph While he was doing experiments on Maxwell’s Theory of Electromagnetic Waves, Heinrich Hertz noticed that some metallic surfaces lost their negative charges when they were exposed to ultraviolet light. This phenomenon was called the photoelectric effect and the electrons emitted from the metal’s surface were termed photoelectrons. Many experiments on photoelectricity were performed using a Cathode Ray Tube (CRT). If the voltage applied is reversed then the anode becomes negative. If the potential is then increased it is possible to ‘turn back’(stop) the photoelectric current. The experiments yielded several important results: 1. Electrons are only emitted when the frequency of the incident light is above a certain value called the threshold frequency. At this frequency or above this the frequency magnitude of the photoelectric current varies directly as the intensity of the light. More intense light more photoelectrons (current) 2. Below the threshold frequency there is no photoelectric current regardless of the intensity of the light. 3. The threshold frequency is different for different kinds of cathodes (metals) 4. As a retarding potential is increased the photoelectric current decreases independent of the intensity of the light. From this scientists learned that photoelectrons have different amounts of energy. A potential can be reached that completely stops the photoelectric current. This potential difference is called the cut-off potential or stopping voltage, Vstop. 5. Different frequencies of light (above the threshold frequency) have different stopping potentials. The higher the frequency of light the higher the stopping potential. The stopping potential is related to the maximum kinetic energy of the photoelectrons. (The slope of all three graphs is the same – later known to be planck’s constant) 6. When photoelectrons are emitted the electrons are released immediately even for weak light sources. (There was no time needed to build up energy absorption.) Albert Einstein proposed a new theory to account for the experimental results. He suggested that light energy is not transmitted as a continuous wave but rather as small packets of energy called photons (quanta). The amount of energy in each photon was a definite amount determined by the frequency of the light. The amount of energy in a photon was given by E=hf. Einstein reasoned that the electrons near the surface of a metal (cathode) needed some energy to escape from the surface. Any extra energy they received showed up in the electrons as kinetic energy. (measured by the stopping voltage). Summary: Light Higher frequency (color) Higher intensity (brightness) Photoelectron Higher energy (voltage) More electrons (current) Theory: When a photon hit an electron all the energy of the photon was given to the electron. If this energy was NOT sufficient for the electron to escape it was re-emitted by the electron. If the energy was sufficient then the electron could escape immediately. If the energy of the photon was more than the electron required then it showed up as kinetic energy in the electron (high velocity). Photon energy escape energy + kinetic energy of electron (conservation of energy) Ephoton= W + Ek electron If the electron just escapes but has no Ek, then Ephoton= W Voltage to stop the current can be used to find the Ek of the electron: Vstop = ΔEk/q Millikan showed that Einstein’s theory was correct in 1916; by showing that the slope of the energy vs. frequency graph for any metal was equal to planck’s constant (h). Pg 259-267 COMPTON EFFECT-MOMENTUM OF A PHOTON Explain, qualitatively and quantitatively, the Compton effect as another example of wave-particle duality, applying the laws of mechanics and of conservation of momentum and energy to photons. Arthur Compton performed an experiment in 1923 in which he directed high-energy X-rays at a metal foil. Compton noted that lower energy (low frequency) X-ray photons were being emitted as well as electrons! Classical electro-magnetic theory could not explain these results. Compton’s theory was that the photon acted like a particle. When a photon struck an electron the photon bounced off the electron but lost some of its energy in the collision. Such a collision would be elastic – that is BOTH momentum and energy would be conserved. Compton used Einstein’s idea that mass was a form of energy (and vice versa) to explain his experimental results. Einstein’s theory of relativity (E=mc2) said that a body with energy E has a mass equivalent of m=E/c2. Because momentum is given by p=mv the momentum of a photon is given by p=E/c2 times v p=E/c but since E=hf for photons, then p=hf/c or p=h/λ Compton’s experiments demonstrated that particle-like property of light or EMR – photons could exhibit mathematically, the conservation of energy and momentum in a collision. Compton’s Scattering Equation: http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/compeq.html where m=mass of the electron θ=angle of scatter of the EMR (x-ray) pg 269-276 De Broglie: (also in Unit 4) In 1924 shortly after the discovery that EMR exhibited both wave and particle behavior, Louis De Broglie suggested that particles might also have both wave and particle behavior. The wavelength of a particle could be related to their momentum. λ = h/p for EMR but p = mv for particles therefore λ = h/(mv) This is called the DeBroglie wavelength!! This seems absurd for large masses to have a wavelength to their motion; and because of their large mass, you can see that mathematically the λ would be near zero. But for extremely small mass particles (like electrons) it can actually be detected that they move with a wave motion (wavelength). Diffraction is a characteristic of wave motion and observed for EMR, but it is also demonstrated by electrons when they pass through a crystal or thin metal foil. They produce a diffraction pattern! Today, the electron microscope uses the wave nature of electrons. However, the wavelength is extremely small. This small wavelength is used to allow us to distinguish the detail so much more clearly with an electron microscope than with a light microscope! Pg 296-298 Theory of relativity: http://topdocumentaryfilms.com/nova-the-elegant-universe/